Regulation of Hindbrain Vascular Development by rps20 in Zebrafish
- PMID: 40710323
- PMCID: PMC12293849
- DOI: 10.3390/cells14141070
Regulation of Hindbrain Vascular Development by rps20 in Zebrafish
Abstract
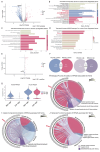
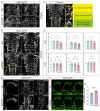
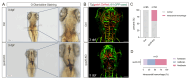
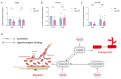
During aging, the brain vasculature undergoes significant deterioration characterized by increased arterial tortuosity, compromised blood-brain barrier integrity, and reduced cerebral blood flow, all of which contribute to various neurological disorders. Thus, understanding the mechanisms underlying aging-related cerebrovascular defects is critical for developing strategies to alleviate aging-associated neurological diseases. In this study, we investigated the role of aging-related genes in brain vascular development using zebrafish as an in vivo model. By thoroughly analyzing scRNA-seq datasets of mid- and old-aged brain vascular endothelial cells (human/mouse), we found ribosomal protein S20 (rps20) significantly down-regulated during aging. qPCR analysis and whole-mount in situ hybridization validated a high expression of rps20 during early zebrafish development, which progressively decreased in adult and aged zebrafish brains. Functional studies using the CRISPR/Cas9-mediated knockout of rps20 revealed an impaired growth of central arteries in the hindbrain and a marked increased intracranial hemorrhage incidence. Mechanistically, qPCR analysis demonstrated a significant downregulation of vegfa, cxcl12b, and cxcr4a, key signaling molecules required for hindbrain vascular development, in rps20-deficient embryos. In conclusion, our findings demonstrate that rps20 is essential for proper brain vascular development and the maintenance of vascular homeostasis in zebrafish, revealing a novel mechanism by which aging-related genes regulate brain vascular development. This study provides new insights that may aid in understanding and treating aging-associated vascular malformations and neurological pathologies.
Keywords: aging; brain vasculature; rps20; vascular permeability; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

