Natural Killer Cell and Extracellular Vesicle-Based Immunotherapy in Thyroid Cancer: Advances, Challenges, and Future Perspectives
- PMID: 40710340
- PMCID: PMC12293766
- DOI: 10.3390/cells14141087
Natural Killer Cell and Extracellular Vesicle-Based Immunotherapy in Thyroid Cancer: Advances, Challenges, and Future Perspectives
Abstract
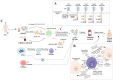
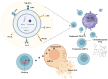
Thyroid cancer, the most frequently occurring endocrine neoplasm, comprises a heterogeneous group of histological subtypes, spanning from the indolent papillary thyroid carcinoma (PTC) to the rapidly progressive and lethal anaplastic thyroid carcinoma (ATC). Although conventional therapies, such as surgery and radioactive iodine (RAI), are effective for differentiated thyroid cancers, treatment resistance and poor prognosis remain major challenges in advanced and undifferentiated forms. In current times, growing attention has been directed toward the potential of Natural Killer (NK) cells as a promising immunotherapeutic avenue. These innate immune cells are capable of direct cytotoxicity against tumor cells, but their efficiency is frequently compromised by the immunosuppressive tumor microenvironment (TME), which inhibits NK cell activation, infiltration, and persistence. This review explores the dynamic interaction between NK cells and the TME in thyroid cancer, detailing key mechanisms of immune evasion, including the impact of suppressive cytokines, altered chemokine landscapes, and inhibitory ligand expression. We further discuss latest advancements in NK cell-based immunotherapies, including strategies for ex vivo expansion, genetic modification, and combinatorial approaches with checkpoint inhibitors or cytokines. Additionally, emerging modalities, such as NK cell-derived extracellular vesicles, are addressed. By combining mechanistic insights with advancing therapeutic techniques, this review provides a comprehensive perspective on NK cell-based interventions and their future potential in improving outcomes for patients with thyroid cancer.
Keywords: CAR-NK cells; immunotherapy; natural killer (NK) cells; thyroid cancer; tumor microenvironment.
Conflict of interest statement
All authors report no relevant conflicts of interest for this article.
Figures
Similar articles
-
Cancer cell-derived extracellular vesicles: a potential target for overcoming tumor immunotherapy resistance and immune evasion strategies.Front Immunol. 2025 Jun 12;16:1601266. doi: 10.3389/fimmu.2025.1601266. eCollection 2025. Front Immunol. 2025. PMID: 40574844 Free PMC article. Review.
-
Anti-tumor effects on tumor-infiltrating natural killer cells by localized ablative immunotherapy and immune checkpoint inhibitors: An integrated and comparative study using scRNAseq analysis.Cancer Lett. 2025 Sep 1;627:217825. doi: 10.1016/j.canlet.2025.217825. Epub 2025 May 26. Cancer Lett. 2025. PMID: 40436260
-
Harnessing Natural Killer Cells for Lung Cancer Therapy.Cancer Res. 2023 Oct 13;83(20):3327-3339. doi: 10.1158/0008-5472.CAN-23-1097. Cancer Res. 2023. PMID: 37531223 Review.
-
NK cell-based immunotherapy in hepatocellular carcinoma: An attractive therapeutic option for the next decade.Cell Signal. 2024 Dec;124:111405. doi: 10.1016/j.cellsig.2024.111405. Epub 2024 Sep 12. Cell Signal. 2024. PMID: 39260532 Review.
-
From natural defenders to therapeutic warriors: NK cells in HIV immunotherapy.Immunotherapy. 2025 Feb;17(2):133-145. doi: 10.1080/1750743X.2025.2460965. Epub 2025 Feb 5. Immunotherapy. 2025. PMID: 39905963 Review.
References
-
- Oh J.M., Ahn B.C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. 2021;11:6251–6277. doi: 10.7150/thno.57689. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

