Fundus Autofluorescence in Inherited Retinal Disease: A Review
- PMID: 40710345
- PMCID: PMC12293359
- DOI: 10.3390/cells14141092
Fundus Autofluorescence in Inherited Retinal Disease: A Review
Abstract
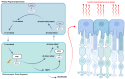
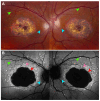
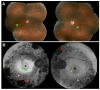
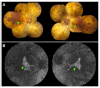
Fundus autofluorescence (FAF) is a non-invasive retinal imaging technique that helps visualize naturally occurring fluorophores, such as lipofuscin, and provides valuable insight into retinal diseases-particularly inherited retinal diseases (IRDs). FAF is especially useful in detecting subclinical or early-stage IRDs and in monitoring disease progression over time. In Stargardt disease, areas of decreased autofluorescence correlate with disease progression and have been proposed as a biomarker for future clinical trials. FAF can also help differentiate Stargardt disease from other macular dystrophies. In retinitis pigmentosa, hyperautofluorescent rings are a common feature on FAF and serve as an important marker for disease monitoring, especially as changes align with those seen on other imaging modalities. FAF is valuable in tracking progression of choroideremia and may help identify disease carrier status. FAF has also improved the characterization of mitochondrial retinopathies such as maternally inherited diabetes and deafness. As a rapid and widely accessible imaging modality, FAF plays a critical role in both diagnosis and longitudinal care of patients with IRDs.
Keywords: fundus autofluorescence; inherited retinal diseases.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Accuracy of fundus autofluorescence imaging for the diagnosis and monitoring of retinal conditions: a systematic review.Health Technol Assess. 2016 Apr;20(31):1-108. doi: 10.3310/hta20310. Health Technol Assess. 2016. PMID: 27115052 Free PMC article.
-
Flavoprotein Fluorescence Imaging in Stargardt Disease: Linking Metabolic Stress to Structural Damage.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):12. doi: 10.1167/iovs.66.11.12. Invest Ophthalmol Vis Sci. 2025. PMID: 40767444 Free PMC article.
-
Blue Light and Green Light Fundus Autofluorescence, Complementary to Optical Coherence Tomography, in Age-Related Macular Degeneration Evaluation.Diagnostics (Basel). 2025 Jul 2;15(13):1688. doi: 10.3390/diagnostics15131688. Diagnostics (Basel). 2025. PMID: 40647687 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Delori F.C., Dorey C.K., Staurenghi G., Arend O., Goger D.G., Weiter J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995;36:718–729. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

