Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis
- PMID: 40710347
- PMCID: PMC12294002
- DOI: 10.3390/cells14141094
Centriole Duplication at the Crossroads of Cell Cycle Control and Oncogenesis
Abstract
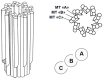
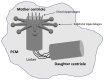
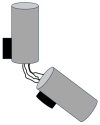
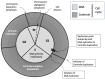
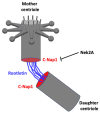
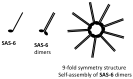
Centriole duplication is a vital process for cellular organisation and function, underpinning essential activities such as cell division, microtubule organisation and ciliogenesis. This review summarises the latest research on the mechanisms and regulatory pathways that control this process, focusing on important proteins such as polo-like kinase 4 (PLK4), SCL/TAL1 interrupting locus (STIL) and spindle assembly abnormal protein 6 (SAS-6). This study examines the complex steps involved in semi-conservative duplication, from initiation in the G1-S phase to the maturation of centrioles during the cell cycle. Additionally, we will explore the consequences of dysregulated centriole duplication. Dysregulation of this process can lead to centrosome amplification and subsequent chromosomal instability. These factors are implicated in several cancers and developmental disorders. By integrating recent study findings, this review emphasises the importance of centriole duplication in maintaining cellular homeostasis and its potential as a therapeutic target in disease contexts. The presented findings aim to provide a fundamental understanding that may inform future research directions and clinical interventions related to centriole biology.
Keywords: cell cycle control; centriole duplication; chromosomal instability; ciliopathies; oncogenesis.
Conflict of interest statement
The author declares no conflicts of interest.
Figures



Similar articles
-
Binding of CEP152 to PLK4 stimulates kinase activity to promote centriole assembly.Mol Biol Cell. 2025 Jul 1;36(7):br17. doi: 10.1091/mbc.E24-12-0581. Epub 2025 May 15. Mol Biol Cell. 2025. PMID: 40372713
-
14-3-3ε inhibits premature centriole disengagement by inhibiting the activity of Plk1 and separase.J Cell Sci. 2025 Jul 15;138(14):jcs263808. doi: 10.1242/jcs.263808. Epub 2025 Jul 18. J Cell Sci. 2025. PMID: 40576425 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Role of Polo-like Kinases Plk1 and Plk4 in the Initiation of Centriole Duplication-Impact on Cancer.Cells. 2022 Feb 24;11(5):786. doi: 10.3390/cells11050786. Cells. 2022. PMID: 35269408 Free PMC article. Review.
-
The PLK4-STIL-SAS-6 module at the core of centriole duplication.Biochem Soc Trans. 2016 Oct 15;44(5):1253-1263. doi: 10.1042/BST20160116. Biochem Soc Trans. 2016. PMID: 27911707 Free PMC article. Review.
References
-
- Boveri T. Zur Frage der Entstehung Maligner Tumoren. Volume 40. Gustav Fischer; Jena, Germany: 1914. pp. 857–859.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

