Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development
- PMID: 40710350
- PMCID: PMC12293387
- DOI: 10.3390/cells14141097
Role of Cellular Senescence in IUGR: Impact on Fetal Morbidity and Development
Abstract
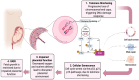
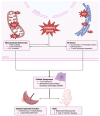
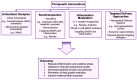
Intrauterine growth restriction (IUGR) is a critical challenge in perinatal medicine and is associated with significant morbidity and mortality. This review explores the intricate involvement of early developmental senescence in IUGR. We highlight the dual role of cellular senescence in both normal development and pathological conditions, emphasizing the need for further research to elucidate these mechanisms and develop targeted interventions. We discuss how oxidative stress and mitochondrial dysfunction affect senescence determinants. We present emerging therapeutic strategies aimed at targeting senescence and inflammation in the placenta. We also introduce Rytvela, an interleukin-1 (IL-1) receptor modulator developed in our laboratory, which selectively attenuates pro-inflammatory signaling while preserving essential immune responses, which in turn mitigate senescence. By addressing senescence-related dysfunctions, such interventions may improve placental performance and fetal outcomes, opening up new directions for the clinical management of IUGR.
Keywords: IUGR; Rytvela; development; oxidative stress; senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Suhag A., Berghella V. Intrauterine Growth Restriction (IUGR): Etiology and Diagnosis. Curr. Obstet. Gynecol. Rep. 2013;2:102–111. doi: 10.1007/s13669-013-0041-z. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

