IP3R2-Mediated Astrocytic Ca2+ Transients Are Critical to Sustain Modulatory Effects of Locomotion on Neurons in Mouse Somatosensory Cortex
- PMID: 40710356
- PMCID: PMC12293227
- DOI: 10.3390/cells14141103
IP3R2-Mediated Astrocytic Ca2+ Transients Are Critical to Sustain Modulatory Effects of Locomotion on Neurons in Mouse Somatosensory Cortex
Abstract
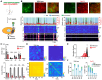
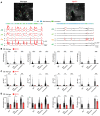
Accumulating studies have shown that astrocytes are essential for regulating neurons at both synaptic and circuit levels. The main mechanism of brain astrocytic intracellular Ca2+ activity is through the release of Ca2+ via the inositol 1,4,5-trisphosphate receptor type 2 (IP3R2) from the endoplasmic reticulum (ER). Studies using IP3R2 knockout mouse models (Itpr2-/-) have shown that eliminating IP3R2 leads to a significant reduction in astrocytic Ca2+ activity However, there is ongoing controversy regarding the effect of this IP3R2-dependent reduction in astrocytic Ca2+ transients on neuronal activity. In our study, we employed dual-color two-photon Ca2+ imaging to study astrocytes and neurons simultaneously in vibrissa somatosensory cortex (vS1) in awake-behaving wild-type and Itpr2-/- mice. We systematically characterized and compared both recorded astrocytic and neuronal Ca2+ activities in wild-type and Itpr2-/- mice during various animal behaviors, particularly during the transition period from stillness to locomotion. We report that vS1 astrocytic Ca2+ elevation in both wild-type and Itpr2-/- mice was significantly modulated by free whisking and locomotion. However, vS1 neurons were only significantly modulated by locomotion in wild-type mice, but not in Itpr2-/- mice. Our study suggests a non-synaptic modulatory mechanism on functions of astrocytic IP3R2-dependent Ca2+ transients to local neurons.
Keywords: Ca2+ signaling; IP3R2; astrocytes; genetically encoded calcium indicator; locomotion; neuromodulation; neuro–glial interaction; two-photon imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cerebral blood flow is modulated by astrocytic cAMP elevation independently of IP3R2-mediated Ca2+ signaling in mice.Proc Natl Acad Sci U S A. 2025 Jul 8;122(27):e2422069122. doi: 10.1073/pnas.2422069122. Epub 2025 Jul 1. Proc Natl Acad Sci U S A. 2025. PMID: 40591593
-
Astrocyte Store-Released Calcium Modulates Visual Cortex Synapse Development and Circuit Function.bioRxiv [Preprint]. 2025 Jul 22:2025.07.20.665758. doi: 10.1101/2025.07.20.665758. bioRxiv. 2025. PMID: 40777341 Free PMC article. Preprint.
-
Disruption of IP₃R2-mediated Ca²⁺ signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke.Cell Calcium. 2015 Dec;58(6):565-76. doi: 10.1016/j.ceca.2015.09.004. Epub 2015 Sep 25. Cell Calcium. 2015. PMID: 26433454 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

