Zebrafish as a Model for Translational Immuno-Oncology
- PMID: 40710421
- PMCID: PMC12298638
- DOI: 10.3390/jpm15070304
Zebrafish as a Model for Translational Immuno-Oncology
Abstract
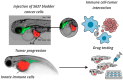
Despite remarkable progress in cancer immunotherapy, many agents that show efficacy in murine or in vitro models fail to translate clinically. Zebrafish (Danio rerio) have emerged as a powerful complementary model that addresses several limitations of traditional systems. Their optical transparency, genetic tractability, and conserved immune and oncogenic signaling pathways enable high-resolution, real-time imaging of tumor-immune interactions in vivo. Importantly, zebrafish offer a unique opportunity to study the core mechanisms of health and sickness, complementing other models and expanding our understanding of fundamental processes in vivo. This review provides an overview of zebrafish immune system development, highlighting tools for tracking innate and adaptive responses. We discuss their application in modeling immune evasion, checkpoint molecule expression, and tumor microenvironment dynamics using transgenic and xenograft approaches. Platforms for high-throughput drug screening and personalized therapy assessment using patient-derived xenografts ("zAvatars") are evaluated, alongside limitations, such as temperature sensitivity, immature adaptive immunity in larvae, and interspecies differences in immune responses, tumor complexity, and pharmacokinetics. Emerging frontiers include humanized zebrafish, testing of next-generation immunotherapies, such as CAR T/CAR NK and novel checkpoint inhibitors (LAG-3, TIM-3, and TIGIT). We conclude by outlining the key challenges and future opportunities for integrating zebrafish into the immuno-oncology pipeline to accelerate clinical translation.
Keywords: patient-derived xenografts (zAvatars); translational oncology; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Olson B., Li Y., Lin Y., Liu E.T., Patnaik A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018;8:1358–1365. doi: 10.1158/2159-8290.CD-18-0044. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

