Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars
- PMID: 40710546
- PMCID: PMC12299403
- DOI: 10.3390/metabo15070447
Multi-Omics Analysis of Gut Microbiota and Sperm Quality in Tibetan Breeding Boars
Abstract
Background/objectives: Reproductive efficiency in breeding boars critically impacts swine industry productivity, with sperm quality being multifactorially regulated by gut microbiota. This study aimed to elucidate the microbiota-metabolite interactions underlying sperm quality differences in Tibetan boars.
Methods: Integrated 16S rRNA sequencing and untargeted metabolomics were performed on fecal and semen samples from eight healthy Tibetan boars (31-33 months old), stratified into low-semen (CJ) and high-semen utilization (HJ) groups. Analyses included sperm quality assessment, microbial profiling, and metabolic pathway enrichment.
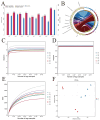
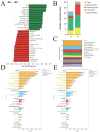
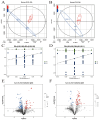
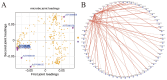
Results: The HJ group exhibited significantly enhanced sperm motility and semen utilization rates (p < 0.05). Gut microbiota composition differed markedly, with Firmicutes and Proteobacteria enriched in HJ boars. Metabolomics identified key metabolites positively correlated with sperm quality (e.g., butyrate, phenyllactic acid), while lithocholic acid showed negative associations. KEGG analysis revealed predominant involvement in butanoate metabolism and bile acid biosynthesis. Core microbiota (e.g., Ruminococcus) modulated sperm quality through short-chain fatty acid networks and bile acid homeostasis.
Conclusions: Gut microbiota regulated the sperm microenvironment via a "metabolic-immune" dual pathway mediated by the gut-testis axis. These findings establish a theoretical basis for probiotic or metabolite-targeted strategies to improve boar reproductive performance.
Keywords: 16S rRNA; gut microbiota; gut–testis axis; metabolomics; sperm quality.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Characteristics of gut microbiota in patients with asthenozoospermia: a Chinese pilot study.BMC Microbiol. 2024 Jan 15;24(1):22. doi: 10.1186/s12866-023-03173-5. BMC Microbiol. 2024. PMID: 38225541 Free PMC article.
-
Improvement of semen quality in Longyou Partridge Chicken by dietary N-acetyl-L-glutamic acid and its mechanism study.Poult Sci. 2025 Aug;104(8):105234. doi: 10.1016/j.psj.2025.105234. Epub 2025 Apr 30. Poult Sci. 2025. PMID: 40349463 Free PMC article.
-
Multi-omics analysis reveals the efficacy of two probiotic strains in managing feline chronic kidney disease through gut microbiome and host metabolome.Front Vet Sci. 2025 Jun 18;12:1590388. doi: 10.3389/fvets.2025.1590388. eCollection 2025. Front Vet Sci. 2025. PMID: 40607345 Free PMC article.
-
Pharmaco-psychiatry and gut microbiome: a systematic review of effects of psychotropic drugs for bipolar disorder.Microbiology (Reading). 2025 Jun;171(6):001568. doi: 10.1099/mic.0.001568. Microbiology (Reading). 2025. PMID: 40528728 Free PMC article. Review.
-
The Androbactome and the Gut Microbiota-Testis Axis: A Narrative Review of Emerging Insights into Male Fertility.Int J Mol Sci. 2025 Jun 27;26(13):6211. doi: 10.3390/ijms26136211. Int J Mol Sci. 2025. PMID: 40649988 Free PMC article. Review.
References
-
- Zhao Y., Tian M., Cheng Z., Wang J., Ren Z. DNA Methylation may be a testicular plateau adaptation in Tibetan pig. J. Appl. Anim. Res. 2021;49:62–67. doi: 10.1080/09712119.2021.1882465. - DOI
Grants and funding
- 2022YFD1600900/National Key Research and Development Project
- XZ202501ZY0147/Tibet Autonomous Region Science and Technology Program
- 2022YFD1600900/The research presented here was funded by National Key Research and Development Project
- XZ202501ZY0147/Tibet Autonomous Region Science and Technology Program
- 2023TC055/China Agricultural University-Tibet Agriculture and Animal Husbandry College Scientific Research Joint Fund Project
LinkOut - more resources
Full Text Sources

