The Influence of Calcium Ions and pH on Fluoride Release from Commercial Fluoride Gels in an In Vitro Study
- PMID: 40710648
- PMCID: PMC12296090
- DOI: 10.3390/gels11070486
The Influence of Calcium Ions and pH on Fluoride Release from Commercial Fluoride Gels in an In Vitro Study
Abstract
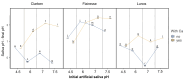
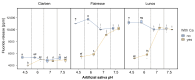
Fluoride gels are widely used in dental prophylaxis due to their proven ability to prevent demineralization and promote remineralization of hard dental tissues. However, the effectiveness of fluoride release from such gels may be significantly influenced by environmental factors such as pH and the presence of calcium ions. This in vitro study aimed to evaluate how these variables affect fluoride ion release from three commercially available fluoride gels-Clarben, Flairesse, and Lunos. The gels were incubated in artificial saliva of varying pH levels (4.5, 6.0, 7.0, and 7.5) with and without the addition of calcium, as well as in other water-based media-tap water, deionized water, and 0.9% NaCl solution. Fluoride release and changes in pH were measured and statistically analyzed using a multifactorial ANOVA. The results revealed that fluoride release was highest in calcium-free environments and at neutral to slightly alkaline pH, while the presence of calcium significantly reduced fluoride availability. Among the tested products, Flairesse and Lunos exhibited sensitivity to calcium's presence, unlike Clarben. Fluoride release was generally higher in water than in artificial saliva. Additionally, all gels induced a decrease in pH, which varied depending on the initial pH and calcium content. These findings underline the importance of environmental conditions in optimizing the clinical efficacy of fluoride gel applications.
Keywords: calcium interference; dental biomaterials; fluoride gels; fluoride release; in vitro incubation; statistical analysis.
Conflict of interest statement
The authors declare no conflicts of interest. Specifically, no conflict of interest or received financial support is declared from the manufacturers of the commercial products used.
Figures




Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Fluoride gels for preventing dental caries in children and adolescents.Cochrane Database Syst Rev. 2015 Jun 15;2015(6):CD002280. doi: 10.1002/14651858.CD002280.pub2. Cochrane Database Syst Rev. 2015. PMID: 26075879 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Slow-release fluoride devices for the control of dental decay.Cochrane Database Syst Rev. 2018 Mar 1;3(3):CD005101. doi: 10.1002/14651858.CD005101.pub4. Cochrane Database Syst Rev. 2018. PMID: 29495063 Free PMC article.
Cited by
-
Fluoride Content in Infusions of Selected Teas Available on the Polish Market-An In Vitro Study.Foods. 2025 Jul 12;14(14):2452. doi: 10.3390/foods14142452. Foods. 2025. PMID: 40724273 Free PMC article.
References
-
- Kosior P., Dobrzyński M., Korczyński M., Herman K., Czajczyńska-Waszkiewicz A., Kowalczyk-Zając M., Piesiak-Pańczyszyn D., Fita K., Janeczek M. Long-Term Release of Fluoride from Fissure Sealants—In Vitro Study. J. Trace Elem. Med. Biol. 2017;41:107–110. doi: 10.1016/j.jtemb.2017.02.014. - DOI - PubMed
-
- Kaczmarek U., Jackowska T., Mielnik-Błaszczak M., Jurczak A., Olczak-Kowalczyk D. Individualised Caries Prevention with Fluoride in Children and Adolescents–Recommendations of Polish Experts. Nowa Stomatol. 2019;24:70–85. doi: 10.25121/NS.2019.24.2.70. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

