Clinical Safety and Efficacy of Hyaluronic Acid-Niacinamide-Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening
- PMID: 40710660
- PMCID: PMC12294899
- DOI: 10.3390/gels11070495
Clinical Safety and Efficacy of Hyaluronic Acid-Niacinamide-Tranexamic Acid Injectable Hydrogel for Multifactorial Facial Skin Quality Enhancement with Dark Skin Lightening
Abstract
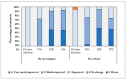
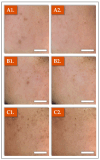
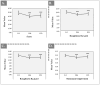
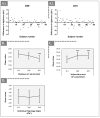
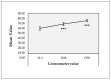
Facial aging is a complex process manifesting as skin hyperpigmentation, textural irregularities, and a diminished elasticity, hydration, and evenness of tone. The escalating demand for minimally invasive aesthetic interventions has driven the development of advanced hydrogel-based injectable formulations. This clinical study assessed the safety and efficacy of Hydragel A1, an injectable hydrogel containing hyaluronic acid (HA), niacinamide, and tranexamic acid (TXA), designed to simultaneously address multiple facets of facial skin aging. A cohort of 49 female participants underwent a series of objective and subjective assessments, including the Global Aesthetic Improvement Scale (GAIS), instrumental measurements (Antera 3D, Chromameter, Cutometer, Dermascan, Corneometer), and standardized photographic documentation at baseline (Day 0) and 14, 28, and 70 days post-treatment. The results demonstrated statistically significant improvements in skin hydration, texture, elasticity, and pigmentation following Hydragel A1 administration. Notably, no serious adverse events or significant injection site reactions were observed, confirming the favorable safety profile of the investigated device. Collectively, these findings underscore the potential of a combined HA, niacinamide, and TXA injectable formulation to provide a comprehensive approach to facial skin rejuvenation, effectively targeting multiple aging-related mechanisms.
Keywords: aesthetic medicine; clinical trial; dermal fillers; facial aging; hyaluronic acid; hyperpigmentation; injectable therapeutics; niacinamide; skin texture; tranexamic acid.
Conflict of interest statement
Authors S.H., K.L., A.P., and C.M. were employed by LOUNA REGENERATIVE SA (Geneva, Switzerland) during the course of this study. Author A.E.L. was employed by LAM Biotechnologies SA (Epalinges, Switzerland) and by TEC-PHARMA SA (Bercher, Switzerland) during the course of this study. The remaining authors declare no conflicts of interest for this study.
Figures



Similar articles
-
A Pilot One and Two-Year Prospective, Blinded Clinical Evaluation of Efficacy, and Safety of Combined Treatment With Crosslinked Hyaluronic Acid Dermal Filler and Barbed Polydioxanone Suspension Threads for Mid-Face Contour Enhancement.J Cosmet Dermatol. 2025 Feb;24(2):e16700. doi: 10.1111/jocd.16700. Epub 2024 Dec 18. J Cosmet Dermatol. 2025. PMID: 39692727 Free PMC article. Clinical Trial.
-
Noninferiority Study Comparing the Efficacy and Safety of a New Hyaluronic Acid (HA) Filler Containing Lidocaine With an Existing HA Filler for the Treatment of Nasolabial Fold Wrinkles: A Randomized, Double-Blind, Split-Face Trial.J Cosmet Dermatol. 2025 Jul;24(7):e70309. doi: 10.1111/jocd.70309. J Cosmet Dermatol. 2025. PMID: 40620129 Free PMC article. Clinical Trial.
-
Facial Skin Quality Improvement After Treatment With CPM-HA20G: Clinical Experience in Korea.J Cosmet Dermatol. 2025 Jan;24(1):e16795. doi: 10.1111/jocd.16795. J Cosmet Dermatol. 2025. PMID: 39844659 Free PMC article. Clinical Trial.
-
Interventions for acne scars.Cochrane Database Syst Rev. 2016 Apr 3;4(4):CD011946. doi: 10.1002/14651858.CD011946.pub2. Cochrane Database Syst Rev. 2016. PMID: 27038134 Free PMC article.
-
Permanent Facial Fillers: Addressing Complications and Advancing Solutions.Aesthetic Plast Surg. 2025 Apr;49(7):2137-2139. doi: 10.1007/s00266-024-04104-z. Epub 2024 May 8. Aesthetic Plast Surg. 2025. PMID: 38720100 Review.
References
-
- Hsin S., Lourenço K., Porcello A., Marques C., Rodriguez C., Raffoul W., Scaletta C., Abdel-Sayed P., Hadjab B., Applegate L.A., et al. Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients. Cosmetics. 2024;11:168. doi: 10.3390/cosmetics11050168. - DOI
-
- Karrabi M., Mansournia M.A., Sharestanaki E., Abdollahnejad Y., Sahebkar M. Clinical evaluation of efficacy and tolerability of cysteamine 5% cream in comparison with tranexamic acid mesotherapy in subjects with melasma: A single-blind, randomized clinical trial study. Arch. Dermatol. Res. 2021;313:539–547. doi: 10.1007/s00403-020-02133-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources

