MOFs-Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel
- PMID: 40710676
- PMCID: PMC12294706
- DOI: 10.3390/gels11070514
MOFs-Combining Fully Synthetic Injectable Hydrogel Scaffolds Exhibiting Higher Skeletal Muscle Regenerative Efficiency than Matrigel
Abstract
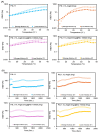
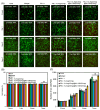
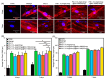
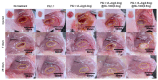
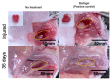
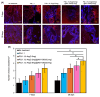
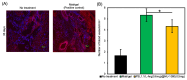
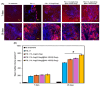
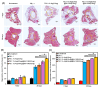
Due to its sarcoma-derived origin and the associated carcinogenic risks, as well as its lack of tissue-specific extracellular matrix biochemical cues, the use of the injectable gel scaffold Matrigel is generally restricted to research applications. Therefore, the development of new fully synthetic injectable gel scaffolds that exhibit performance comparable to Matrigel is a high priority. In this study, we developed a novel fully synthetic injectable gel scaffold by combining a biodegradable PLGA-PEG-PLGA copolymer, clay nanoparticle LAPONITE®, and L-arginine-loaded metal-organic frameworks (NU-1000) at the nano level. An aqueous solution of the developed hybrid scaffold (PLGA-PEG-PLGA/LAPONITE®/L-Arg@NU-1000) exhibited rapid sol-gel transition at body temperature following simple injection and formed a continuous bulk-sized gel, demonstrating good injectability. Long-term sustained slow release of L-arginine from the resultant gels can be achieved because NU-1000 is a suitable reservoir for L-arginine. PLGA-PEG-PLGA/LAPONITE®/L-Arg@NU-1000 hybrid gels exhibited good compatibility with and promoted the growth of human skeletal muscle satellite cells. Importantly, in vivo experiments using skeletal muscle injury model mice demonstrated that the tissue regeneration efficiency of PLGA-PEG-PLGA/LAPONITE®/L-Arg@NU-1000 gels is higher than that of Matrigel. Specifically, we judged the higher tissue regeneration efficacy of our gels by histological analysis, including MYH3 immunofluorescent staining, H&E staining, and Masson's trichrome staining. Taken together, these data suggest that novel hybrid hydrogels could serve as injectable hydrogel scaffolds for in vivo tissue engineering and ultimately replace Matrigel.
Keywords: MOFs; injectable hydrogels; scaffolds; skeletal muscle tissue; tissue engineering.
Conflict of interest statement
The authors declare that the commercial products LAPONITE® and PLGA-PEG-PLGA/LAPONITE®/L-Arg@NU-1000 do not involve any conflicts of interest.
Figures







References
-
- Li H., Eddaoudi M., Keeffe M.O., Yaghi O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature. 1999;402:276–279. doi: 10.1038/46248. - DOI
-
- Daglar H., Gulbalkan H.C., Avci G., Aksu G.O., Altundal O.F., Altintas C., Erucar L., Keskin S. Effects of metal-organic framework (MOF) database selection on the assessment of gas storage and separation potentials of MOFs. Angew. Chem. Int. Ed. Engl. 2021;60:7828–7837. doi: 10.1002/anie.202015250. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

