The Anatomy of the Atrioventricular Node
- PMID: 40710771
- PMCID: PMC12295059
- DOI: 10.3390/jcdd12070245
The Anatomy of the Atrioventricular Node
Abstract
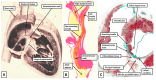
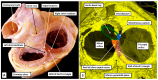
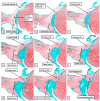
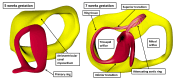
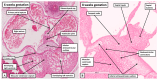
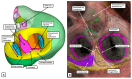
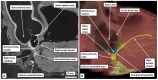
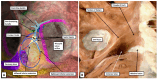
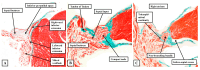
The anatomical arrangement of the atrioventricular node has been likened to a riddle wrapped up in an enigma. There are several reasons for this alleged mystery, not least the marked variability in structure between different species. Lack of detailed knowledge of the location of the node relative to the atrial and ventricular septal structures has also contributed to previous misunderstandings. Recent studies comparing the findings of gross dissection with virtual dissection of living datasets, combined with access to a large number of serially sectioned human and animal hearts, have served to provide the evidence to solve the riddle. We summarise these findings in this review. We explain how the node is located within the atrial walls of the inferior pyramidal space. It becomes the non-branching component of the atrioventricular conduction axis as the axis extends through the plane of atrioventricular insulation to enter the infero-septal recess of the left ventricular outflow tract. The node itself is formed by contributions from the tricuspid and mitral vestibules, with extensive additional inputs from the base of the atrial septum. We show how knowledge of development enhances the appreciation of the arrangements and offers an explanation as to why, on occasion, there can be persisting nodoventricular connections. We discuss the findings relative to the circuits producing atrioventricular re-entry tachycardia. We conclude by emphasising the significance of the variation of the anatomical arrangements within different mammalian species.
Keywords: compact node; fast pathway; inferior extensions; inferior pyramidal space; infero-septal recess; slow pathway.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Brunet J., Cook A.C., Walsh C.L., Cranley J., Tafforeau P., Engel K., Arthurs O., Berruyer C., Burke O’Leary E., Bellier A., et al. Multidimensional analysis of the adult human heart in health and disease using hierarchical phase-contrast tomography. Radiology. 2024;312:e232731. doi: 10.1148/radiol.232731. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

