Viability and Longevity of Human Miniaturized Living Myocardial Slices
- PMID: 40710794
- PMCID: PMC12295330
- DOI: 10.3390/jcdd12070269
Viability and Longevity of Human Miniaturized Living Myocardial Slices
Abstract
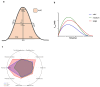
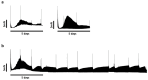
Living myocardial slices (LMSs) have shown great promise in cardiac research, allowing multicellular and complex interplay analyses with disease and patient specificity, yet their wider clinical use is limited by the large tissue sizes usually required. We therefore produced mini-LMSs (<10 mm2) from routine human cardiac surgery specimens and compared them with medium (10-30 mm2) and large (>30 mm2) slices. Size effects on biomechanical properties were examined with mathematical modeling, and viability, contraction profiles, and histological integrity were followed for 14 days. In total, 34 mini-, 25 medium, and 30 large LMS were maintained viable, the smallest measuring only 2 mm2. Peak twitch force proved to be size-independent, whereas time-to-peak shortened as slice area decreased. Downsized LMSs displayed excellent contractile behavior for five to six days, after which a gradual functional decline and micro-architectural changes emerged. These findings confirm, for the first time, that mini-LMSs are feasible and viable, enabling short-term, patient-specific functional studies and pharmacological testing when tissue is scarce.
Keywords: 3D culture systems; experimental methods; in vitro models; living myocardial slices (LMSs); translational cardiology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Fischer C., Milting H., Fein E., Reiser E., Lu K., Seidel T., Schinner C., Schwarzmayr T., Schramm R., Tomasi R., et al. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat. Commun. 2019;10:117. doi: 10.1038/s41467-018-08003-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

