Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management
- PMID: 40710795
- PMCID: PMC12296174
- DOI: 10.3390/jcdd12070270
Navigating Cardiotoxicity in Immune Checkpoint Inhibitors: From Diagnosis to Long-Term Management
Abstract
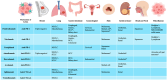
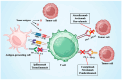
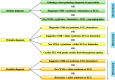
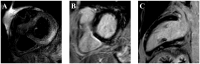
The advent of immune checkpoint inhibitors (ICIs) has revolutionized cancer treatment, significantly improving patient outcomes across multiple malignancies. Nonetheless, these therapies are associated with immune-related adverse effects, including cardiotoxicity, which remains a critical concern. This review provides a comprehensive analysis of ICI-related cardiotoxicity, encompassing its pathophysiological mechanisms, risk factors, diagnostic modalities, and management strategies. The onset of cardiotoxicity varies widely, ranging from acute myocarditis to long-term cardiovascular complications. Early identification through clinical assessment, biomarkers, and advanced imaging techniques is crucial for timely intervention. Management strategies include high-dose corticosteroids, other immunosuppressive agents, and supportive therapies, with a focus on balancing oncologic efficacy and cardiovascular safety. Additionally, rechallenging patients with ICIs following cardiotoxic events remains a complex clinical decision requiring multidisciplinary evaluation. As immunotherapy indications expand to include high-risk populations in a curative setting too, optimizing screening, prevention, and treatment strategies is essential to mitigate cardiovascular risks. A deep understanding of the molecular and clinical aspects of ICI-related cardiotoxicity will enhance patient safety and therapeutic decision-making, underscoring the need for ongoing research in this rapidly evolving field.
Keywords: cardiovascular toxicity; diagnosis; immune checkpoint inhibitors; myocarditis; treatment.
Conflict of interest statement
Pagnesi M. has received personal fees from Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Novartis, Roche Diagnostics, and Vifor Pharma. All other authors declare no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

