NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats
- PMID: 40710983
- PMCID: PMC12299122
- DOI: 10.3390/toxics13070538
NOX2/NLRP3-Inflammasome-Dependent Microglia Activation Promotes As(III)-Induced Learning and Memory Impairments in Developmental Rats
Abstract
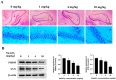
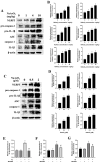
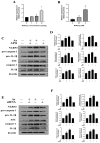
Inorganic arsenic [As(III) and As(V)] is a pervasive environmental contaminant in groundwater systems, early-life exposure to which is associated with an impaired cognitive ability and an increased risk of neurobehavioral disorders. Although the effect of As(III) on the neurons is well studied, the involvement of the microglia remains unclear. In this study, the effects of sodium arsenite (NaAsO2) on microglial activation and the underlying NLRP3 inflammasome mechanism were determined. Pregnant rats were gavaged with NaAsO2 (0, 1, 4, and 10 mg/kg body weight), which dissociates in aqueous solutions into bioactive arsenite species [As(OH)3], from gestational day 1 (GD1) to postnatal day 21 (PND21). The results showed that As(III) induces learning and memory impairments and microglial activation in the hippocampus of offspring rats (PND21). Increased expression of NLRP3, the activation of caspase-1, and the production of interleukin-1β were observed in both the hippocampus of As(III)-exposed offspring rats and As(III)-exposed microglial BV2 cells under culture conditions. Interestingly, blocking the NLRP3 inflammasome using MCC950 mitigated its activation. Furthermore, inhibition of NADPH oxidase 2 (NOX2) using apocynin or specific siRNA significantly reduced As(III)-induced microglial NLRP3 inflammasome activation. In addition, inactivation of the microglial NLRP3 inflammasome or NOX2 markedly rescued As(III)-induced neurotoxicity in the hippocampal HT22 cells. Taken together, this study reveals that NOX2/NLRP3-inflammasome-dependent microglial activation promotes As(III)-induced learning and memory impairments in developmental rats.
Keywords: NLRP3 inflammasome; NOX2; inorganic arsenic; learning and memory; microglia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sanders A.P., Desrosiers T.A., Warren J.L., Herring A.H., Enright D., Olshan A.F., Meyer R.E., Fry R.C. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: A semi-ecologic study. BMC Public Health. 2014;14:955. doi: 10.1186/1471-2458-14-955. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

