Nephrotoxicity of New Antibiotics: A Systematic Review
- PMID: 40711050
- PMCID: PMC12299473
- DOI: 10.3390/toxics13070606
Nephrotoxicity of New Antibiotics: A Systematic Review
Abstract
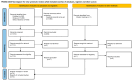
Drug-induced nephrotoxicity is a common and serious problem in clinical practice. We conducted a systematic review of studies reporting nephrotoxicity events associated with antibiotics approved since 2018. The agents assessed included aztreonam/avibactam, cefepime/enmetazobactam, cefiderocol, ceftobiprole, contezolid, gepotidacin, imipenem/cilastatin/relebactam, lascufloxacin, lefamulin, levonadifloxacin, plazomicin, and sulbactam/durlobactam. Literature searches were conducted in PubMed, Scopus, Web of Science, and major pharmacovigilance databases (Vigibase, FAERS, EudraVigilance, EMA, FDA, NMPA, PMDA, and CDSCO) in May 2025, along with reference citation tracking. Studies were included if they reported safety or adverse event data. The risk of bias was assessed using validated tools in accordance with the study design. Out of 2105 potentially relevant records, 74 studies met inclusion criteria, comprising 52 clinical trials, 17 observational studies, 1 registry-based study, 3 case series, and 1 case report. Nephrotoxicity was rarely reported for any of the newly approved antibiotics. No renal adverse events were found in the available studies for aztreonam/avibactam, levonadifloxacin, and contezolid. Most studies were of moderate to high quality; two were classified as low quality. However, nephrotoxicity was inconsistently assessed, with variable definitions and methodologies used. Although current data suggest a low frequency of nephrotoxicity, limitations in study design and reporting preclude firm conclusions. There is a need for post-marketing studies to better characterize renal safety. Clinicians should remain vigilant and continue to monitor for and report renal-related adverse events.
Keywords: aztreonam/avibactam; cefepime/enmetazobactam; cefiderocol; ceftobiprole; contezolid; gepotidacin; imipenem/cilastatin/relebactam; lascufloxacin; lefamulin; levonadifloxacin; plazomicin; sulbactam/durlobactam.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous