Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis
- PMID: 40711151
- PMCID: PMC12299304
- DOI: 10.3390/toxins17070340
Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis
Abstract
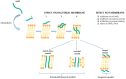
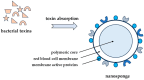
For many years, staphylococci have been detected mainly in infections of the skin and soft tissues, organs, bone inflammations, and generalized infections. Thromboembolic diseases have also become a serious plague of our times, which, as it turns out, are closely related to the toxic effects of staphylococci. Staphylococcus aureus, because of the presence of many different kinds of virulence factors, is capable of manipulating the host's innate and adaptive immune responses. These include toxins and cofactors that activate host zymogens and exoenzymes, as well as superantigens, which are highly inflammatory and cause leukocyte death. Coagulases and staphylokinases can control the host's coagulation system. Nucleases and proteases inactivate various immune defense and surveillance proteins, including complement components, peptides and antibacterial proteins, and surface receptors that are important for leukocyte chemotaxis. On the other hand, secreted toxins and exoenzymes are proteins that disrupt the endothelial and epithelial barrier as a result of cell lysis and disintegration of linking proteins, which ultimately increases the risk of thromboembolism. In this review, we discuss various virulence factors and substances that may inhibit their activity.
Keywords: staphylococcal infections; staphylococcal virulence factors; therapeutic strategies; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Development of a new N-terminomic method to study the pathodegradome of the Staphylococcus aureus V8 protease in human neutrophils.mSystems. 2025 Jul 22;10(7):e0069725. doi: 10.1128/msystems.00697-25. Epub 2025 Jun 25. mSystems. 2025. PMID: 40558068 Free PMC article.
-
Staphylococcus aureus Secreted Toxins and Extracellular Enzymes.Microbiol Spectr. 2019 Mar;7(2):10.1128/microbiolspec.gpp3-0039-2018. doi: 10.1128/microbiolspec.GPP3-0039-2018. Microbiol Spectr. 2019. PMID: 30873936 Free PMC article. Review.
-
Acquisition of daptomycin resistance in patients results in decreased virulence in Drosophila.Infect Immun. 2025 Jun 10;93(6):e0059424. doi: 10.1128/iai.00594-24. Epub 2025 May 23. Infect Immun. 2025. PMID: 40407308 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Alcohol Sanitizer.2023 Aug 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Aug 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30020626 Free Books & Documents.
References
-
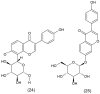
- Olchowik-Grabarek E., Sekowski S., Bitiucki M., Dobrzynska I., Shlyonsky V., Ionov M., Burzynski P., Roszkowska A., Swiecicka I., Abdulladjanova N., et al. Inhibition of interaction between Staphylococcus aureus α-hemolysin and erythrocytes membrane by hydrolysable tannins: Structure-related activity study. Sci. Rep. 2020;10:11168. doi: 10.1038/s41598-020-68030-1. - DOI - PMC - PubMed
-
- Júnior J.G.d.A.S., Coutinho H.D.M., Rodrigues J.P.V., Ferreira V.P.G., Neto J.B.d.A., Costa da Silva M.M., Justino de Araújo A.C., Pereira R.L.S., Alexandre de Aquino P.E., Oliveira–Tintino C.D.d.M., et al. Liposome evaluation in inhibiting pump efflux of NorA of Staphylococcus aureus. Chem. Phys. Lipids. 2022;245:105204. doi: 10.1016/j.chemphyslip.2022.105204. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

