The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission
- PMID: 40711262
- PMCID: PMC12298562
- DOI: 10.3390/vetsci12070602
The Giant Panda Transferrin Receptor Facilitates Feline Parvovirus Infection to Drive Cross-Species Transmission
Abstract
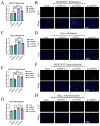
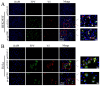
Feline parvovirus (FPV) causes feline panleukopenia, a highly contagious disease in cats, marked by severe leukopenia, biphasic fever, diarrhea, vomiting, and hemorrhagic enteritis. Recently, FPV infection in giant pandas has increased, causing diarrhea and ultimately fatal outcomes, thereby threatening their survival and reproduction. Here, we investigated the transmission of FPV in giant pandas and its interaction with cellular receptors using an FPV strain (pFPV-sc) isolated from giant panda feces. Recombinant feline transferrin receptor 1 (fTfR1) and the giant panda ortholog (gpTfR1) were expressed in non-susceptible HEK293T and HeLa cells, while viral infection levels were measured to determine the effect of gpTfR1 on pFPV-sc replication. The findings indicated that gpTfR1 overexpression in non-susceptible cells significantly enhanced pFPV-sc replication, particularly influencing the viral attachment and internalization stages. Our data further revealed early-stage colocalization between gpTfR1 expression and virus infection, suggesting that gpTfR1 facilitates early viral infection and replication. Taken together, our study provides the first evidence on the mechanism of FPV cross-species infection in giant pandas and elucidates the interaction between gpTfR1 and FPV, which establishes a theoretical basis for the development of preventive and therapeutic strategies, thereby safeguarding the health and survival of giant panda populations from FPV.
Keywords: feline parvovirus; giant panda; transferrin receptor 1; virus replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wang K., Du S.S., Wang Y.Q., Wang S.Y., Luo X.Q., Zhang Y.Y., Liu C.F., Wang H.J., Pei Z.H., Hu G.X. Isolation and identification of tiger parvovirus in captive siberian tigers and phylogenetic analysis of VP2 gene. Infect. Genet. Evol. 2019;75:103957. doi: 10.1016/j.meegid.2019.103957. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

