In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2
- PMID: 40711294
- PMCID: PMC12298981
- DOI: 10.3390/vetsci12070634
In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2
Abstract
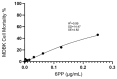
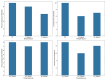
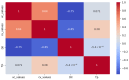
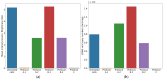
The recent COVID-19 pandemic has prompted the scientific community to prioritize the discovery of preventive methods and new therapeutics, including the investigation of natural compounds with antiviral potential. Fungal secondary metabolites (SMs) represent a promising source of antiviral drugs due to their structural diversity and intrinsic biocompatibility. Herein, the antiviral activity of 6-pentyl-α-pyrone (6PP) against bovine coronavirus (BCoV) has been evaluated in vitro. Considering that BCoV and SARS-CoV-2 are both members of the Betacoronavirus genus and share several key features, BCoV represents a valuable reference model for human coronavirus research. A non-cytotoxic dose of 6PP was used on MDBK cells to evaluate its antiviral activity against BCoV. Different experimental conditions were employed to examine cell monolayer protection both pre- and post-infection, as well as the potential inhibition of viral internalization. Overall, post-infection 6PP treatment reduced viral load and decreased viral internalization. Results were analyzed using viral titration and quantitative PCR, while data interpretation was performed by statistical software tools. This study presents a novel fluorescence quantification approach with high confidence demonstrated by its significant concordance with RT-qPCR results. These data suggest that 6PP could be an effective antiviral agent for BCoV, warranting further investigation of its role in coronavirus inhibition.
Keywords: 6-pentyl-α-pyrone; BCoV; COVID-19; antiviral activity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Structural Features and In Vitro Antiviral Activities of Fungal Metabolites Sphaeropsidins A and B Against Bovine Coronavirus.Int J Mol Sci. 2025 Jul 22;26(15):7045. doi: 10.3390/ijms26157045. Int J Mol Sci. 2025. PMID: 40806195 Free PMC article.
-
Antiviral efficacy of hexane extract of Hypericum gaitii Haines against Chikungunya and SARS-CoV-2 viruses: in vitro and in silico approaches.J Ethnopharmacol. 2025 Jul 10;353(Pt A):120270. doi: 10.1016/j.jep.2025.120270. Online ahead of print. J Ethnopharmacol. 2025. PMID: 40651726
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
References
-
- Padalino B., Cirone F., Zappaterra M., Tullio D., Ficco G., Giustino A., Ndiana L.A., Pratelli A. Factors Affecting the Development of Bovine Respiratory Disease: A Cross-Sectional Study in Beef Steers Shipped From France to Italy. Front. Vet. Sci. 2021;8:627894. doi: 10.3389/fvets.2021.627894. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

