Gedatolisib Combined with Palbociclib and Letrozole in Patients with No Prior Systemic Therapy for Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer
- PMID: 40711480
- PMCID: PMC12485373
- DOI: 10.1158/1078-0432.CCR-25-0992
Gedatolisib Combined with Palbociclib and Letrozole in Patients with No Prior Systemic Therapy for Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer
Abstract
Purpose: Nonclinical evidence demonstrating that estrogen receptor, cyclin-dependent kinases 4 and 6 (CDK4/6), and PI3K/AKT/mTOR (PAM) pathways cross-promote tumor proliferation in hormone receptor-positive (HR+)/HER2- breast cancer cell lines led to the development of CDK4/6 inhibitors and agents inhibiting single PAM pathway nodes to treat HR+/HER2- advanced breast cancer. Simultaneous blockade of the estrogen receptor, CDK4/6, and PAM pathways may optimize antitumor control in the treatment-naïve advanced breast cancer setting. Gedatolisib, a pan-PI3K/mTOR inhibitor, was evaluated as first-line therapy, combined with standard-of-care palbociclib and letrozole, for patients with HR+/HER2- advanced breast cancer.
Patients and methods: Treatment-naïve patients from a phase Ib study with HR+/HER2- advanced breast cancer treated with gedatolisib plus palbociclib and letrozole were analyzed. The primary endpoint of the overall study was investigator-assessed objective response. Secondary endpoints included safety, duration of response, progression-free survival (PFS), and overall survival.
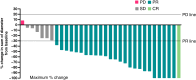
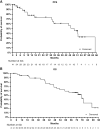
Results: Of 41 patients, all had stage IV disease, 93% had measurable disease, 78% had visceral metastases, and 22% had detectable PIK3CA mutations. The objective response rate was 79% in patients with evaluable disease (N = 33). The median duration of response was 48 months for confirmed responders. The median PFS was 48.4 months, and the median overall survival was 77.3 months. The overall response rate and PFS were comparable in patients with and without PIK3CA mutations. Fewer than 10% discontinued treatment due to treatment-related adverse events. The most frequent grade 3/4 adverse events were neutropenia (61%), rash (39%), and oral stomatitis (29%).
Conclusions: Gedatolisib plus palbociclib and letrozole demonstrated preliminary efficacy in patients with no prior systemic therapy for advanced breast cancer. These results warrant further evaluation of gedatolisib added to standard-of-care, first-line therapy for HR+/HER2- advanced breast cancer.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R. Wesolowski reports nonfinancial support and other support from Celcuity during the conduct of the study as well as nonfinancial support from Celcuity outside the submitted work. H.S. Rugo reports other support from Pfizer and Celcuity, Inc. during the conduct of the study as well as other support from AstraZeneca, Novartis, Lilly, Merck, Roche/Genentech, Gilead, Stemline, and Ambrx and personal fees from Napo and Bristol Myers Squibb outside the submitted work. J.M. Specht reports personal fees from Sensei Biotherapeutics, Scripps Research Institute, BioNTech SE, Daiichi Sankyo, and Boehringer Ingelheim and grants from Merck, Pfizer, Seagen, Genentech, Celcuity, AstraZeneca, Lyell Immunopharma, Carisma Therapeutics, A2 Biotherapeutics, OnKure, and Biocity outside the submitted work. H.S. Han reports other support from Celcuity during the conduct of the study as well as personal fees from Pfizer and Arvinas and other support from Arvinas, Pfizer, Phoenix, Senhwa, Quantum Leap Health, Ellipse Pharma, Regor Therapeutics, Exelixis, BridgeBio Pharma, and Eisai outside the submitted work. P. Kabos reports grants from Celcuity, AstraZeneca, Roche, OnKure, and Eli Lilly during the conduct of the study. U. Vaishampayan reports grants and personal fees from Pfizer during the conduct of the study as well as personal fees from Bristol Myers Squibb, Bayer, AstraZeneca, Novartis, Exelixis, and Janssen and grants and personal fees from Merck outside the submitted work. S.A. Wander reports personal fees from Genentech, Novartis, Hologic, AstraZeneca, Gilead, Foundation Medicine, Veracyte, and Biovica; personal fees and other support from Eli Lilly, Pfizer/Arvinas, Regor Therapeutics, Menarini/Stemline, and Puma Biotechnology; and other support from Sermonix, Phoenix Molecular Designs, 2nd.MD, and Guardant Health outside the submitted work. K. Gogineni reports grants from Pfizer during the conduct of the study as well as institutional grant support for effort as site principal investigator of trials sponsored by BriaCell Therapeutics, ACCRU, Stemline, Novartis, F. Hoffmann-La Roche, SWOG, ECOG-ACRIN, Dana-Farber Cancer Institute, Moffitt, Seattle Genetics, Merck, and Immunomedics. A. Spira reports grants from Roche during the conduct of the study as well as grants from Avenzo Therapeutics outside the submitted work. M. Abu-Khalaf reports other support from Celcuity during the conduct of the study. S.C. Mutka reports other support from Celcuity during the conduct of the study. S. Suzuki reports other support from Celcuity during the conduct of the study as well as other support from Celcuity outside the submitted work. B. Sullivan reports personal fees from Celcuity during the conduct of the study as well as personal fees from Celcuity outside the submitted work. I. Gorbatchevsky reports other support from Celcuity during the conduct of the study. R.M. Layman reports grants, personal fees, and nonfinancial support from Celcuity and Pfizer during the conduct of the study as well as grants and nonfinancial support from Accutar Biotechnology; grants, personal fees, and nonfinancial support from Eli Lilly; grants and personal fees from Novartis and Biotheryx; grants from Puma and Arvinas; and personal fees from Gilead outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300. - PubMed
-
- Lambertini M, Blondeaux E, Bisagni G, Mura S, De Placido S, De Laurentiis M, et al. Prognostic and clinical impact of the endocrine resistance/sensitivity classification according to international consensus guidelines for advanced breast cancer: an individual patient-level analysis from the Mammella InterGruppo (MIG) and Gruppo Italiano Mammella (GIM) studies. eClinicalMedicine 2023;59:101931. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

