TDM-Based Tailored Dosing of Durvalumab in Lung Cancer Patients: A Comprehensive Population Pharmacokinetic-Pharmacoeconomic Evaluation
- PMID: 40711703
- PMCID: PMC12479631
- DOI: 10.1007/s40262-025-01555-8
TDM-Based Tailored Dosing of Durvalumab in Lung Cancer Patients: A Comprehensive Population Pharmacokinetic-Pharmacoeconomic Evaluation
Abstract
Background: The increasing use of immune checkpoint inhibitors, such as durvalumab, places a significant financial burden on healthcare systems, strains hospital capacities, and contributes to environmental concerns.
Objective: We aimed to develop alternative dosing strategies to optimize durvalumab administration, reduce unnecessary drug use, and ensure sustainable cancer care without sacrificing efficacy.
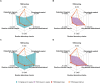
Methods: Using the population pharmacokinetic model developed by the licensing holder, we designed two alternative dosing strategies for non-small cell lung cancer based on therapeutic drug monitoring. Adjustments were made to the dose or administration interval, following regulatory standards for in silico dose optimization. A pharmacoeconomic evaluation was conducted to estimate potential cost savings from a medical perspective.
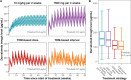
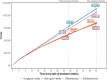
Results: Both alternative strategies achieved high exposure levels, with 98.1-99.0% of patients exceeding a predefined efficacy target, surpassing the 95.4% predicted by the license holder for the approved 10 mg/kg 2-weekly regimen. They also reduced overall drug exposure by 7-24% and eliminated drug wastage, resulting in an average annual cost reduction of €25,163 (22.9%) per patient.
Conclusion: Therapeutic drug monitoring-guided adjustments for durvalumab offer a potentially cost-saving way to optimize drug use, reduce healthcare burdens, and lessen environmental impact while ensuring adequate patient exposure. Our proposal's evidence provides a solid basis for a non-inferiority study.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Financial interest: A.v.d.W. has relationships with AstraZeneca, Boehringer Ingelheim GmbH, Pfizer Inc, F. Hoffmann-La Roche Ltd, Takeda Oncology, Bristol Myers Squibb Co., and Eli Lilly and Company, involving consulting, advisory roles, funding grants, and speaking fees. D.D. has consulting and speaking engagements with Bristol Myers Squibb Co., Merck & Co. Inc., AstraZeneca, Roche, and Pfizer. M.v.d.H. received financial support from the Radboudumc Department of Respiratory Diseases. The parties of interest had no role in the study’s design, data collection, analysis, interpretation, manuscript writing, or the decision to publish. Non-financial interest: A.v.d.W. holds a fiduciary role on the board of the ROS1ders advocacy group. R.t.H. is affiliated with Radboud University Medical Center. The parties of interest had no role in the study’s design, data collection, analysis, interpretation, manuscript writing, or the decision to publish. Editorial Board membership: D.M. is an editorial board member of Clinical Pharmacokinetics. D.M. was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. No interest: The remaining authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Code availability: The population pharmacokinetic model code is included as Supplementary Information. Funding: No funds, grants, or other support was received. Data availability: Data is included as Supplementary Information. Author contributions: F. de Vries: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Validation, Writing—original draft. E.J.F. Franssen: Conceptualization, Supervision, Resources, Writing—review and editing. A.A.J. Smit: Conceptualization, Writing—review and editing. D.J.A.R. Moes: Writing—review and editing. A.J. van der Wekken: Writing—review and editing. T. Oude Munnink: Writing—review and editing. J.J.M.A. Hendrix: Writing—review and editing. D.W. Dumoulin: Writing—review and editing. S.L.W. Koolen: Writing—review and editing. W. Kievit: Methodology, Writing—review and editing. M.M. van den Heuvel: Conceptualization, Writing—review and editing. R. ter Heine: Conceptualization, Methodology, Supervision, Resources, Writing—original draft. All authors have provided their formal approval for this submission.
Figures
References
-
- Cinausero M, Garattini SK, Minisini AM, Valent F, Riosa C, Iacono D, et al. Incremental oncology workload generated by immunotherapy in the first-year of treatment. J Clin Oncol. 2020;38(15_suppl):e14143. 10.1200/JCO.2020.38.15_suppl.e14143.
-
- AstraZeneca. Highlights of prescribing information: Durvalumab (IMFINZI®). 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761069s043lble.... Accessed 10 Sep 2024.
-
- AstraZeneca. Annual Report 2017. 2017. https://www.astrazeneca.com/investor-relations/annual-reports/annual-rep.... Accessed 10 Sep 2024.
-
- European Medicines Agency. Public Assessment Report (EPAR): Durvalumab (IMFINZI®). 2018. https://www.ema.europa.eu/en/documents/assessment-report/imfinzi-epar-pu.... Accessed 10 Sep 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

