A Multicenter Randomized Double-Masked Study Comparing Preservative-free Brimonidine Tartrate Ophthalmic Solution 0.025% with LUMIFY® 0.025% for Ocular Redness in Adults
- PMID: 40711722
- PMCID: PMC12370570
- DOI: 10.1007/s40123-025-01194-z
A Multicenter Randomized Double-Masked Study Comparing Preservative-free Brimonidine Tartrate Ophthalmic Solution 0.025% with LUMIFY® 0.025% for Ocular Redness in Adults
Abstract
Introduction: Ocular redness is common, can affect quality of life, and can be relieved by short-term use of topical adrenergic receptor (AR) agonists. Brimonidine tartrate ophthalmic solution 0.025% (LUMIFY® 0.025%) is the first α2-AR-selective agonist approved for ocular redness. The preservative benzalkonium chloride (BAK) maintains ophthalmic solution sterility, reducing the risk of ocular infections, but may cause symptoms of ocular surface disease (OSD) in some patients. A BAK-free formulation of LUMIFY 0.025% (BTOS-PF 0.025%) could offer a solution for BAK-sensitive patients.
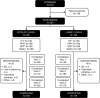
Methods: This randomized, active-controlled, multicenter study aimed to establish non-inferior efficacy of BTOS-PF 0.025% to LUMIFY 0.025% and compare safety. Randomized participants received either formulation instilled as a single drop four times daily, for 4 weeks. The primary endpoint was investigator-assessed ocular redness at 5, 15, 30, 60, 90, 120, 180, and 240 min post-instillation at visit 1 (day 1). Eight hierarchical secondary endpoints included additional post-instillation timepoints at visit 1 and visits 2 and 3 (days 14 and 28), and participant-assessed redness.
Results: BTOS-PF 0.025% was statistically non-inferior to LUMIFY 0.025% for ocular redness reduction across the eight timepoints at visit 1. Efficacy for both formulations was seen as early as 1 min and up to 8 h post-instillation. BTOS-PF 0.025% performed similarly to LUMIFY 0.025% after 14 and 28 days, rebound redness rates were low and similar, and total clearance of ocular redness and participant-evaluated redness were similar. The safety profile of both formulations was similar, with no severe or serious ocular events.
Conclusions: BTOS-PF 0.025% was non-inferior to LUMIFY 0.025% in reducing ocular redness in adults, was well-tolerated, and offers an alternative topical solution, without loss of efficacy or compromised safety, for patients who prefer a preservative-free formulation or are at increased risk of OSD.
Clinical trial registration: ClinicalTrials.gov identifier: NCT05360784. Date of registration: 29 April 2022.
Keywords: Benzalkonium chloride; Brimonidine; Brimonidine tartrate ophthalmic solution 0.025%; Hyperemia; LUMIFY; Ocular redness; Patient preference; Preservative-free; Vasoconstriction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Melissa Toyos is a clinical investigator, speaker and consultant for Bausch + Lomb and Mallinckrodt, a clinical investigator and consultant for Dompé and Sun Pharma, and a clinical investigator for Kala Pharmaceuticals, Glaukos and CBCC Global. Melinda DiVito is an employee of Bausch + Lomb Inc.; Patrick M. Vollmer is a clinical investigator and speaker for Bausch + Lomb; Elisabeth M. Messmer is a speaker for: Alcon, Bausch + Lomb, Bayer, Dompé, Fidia, GlaxoSmithKline, Novartis, OmniVision, Sanofi, Santen GmbH, Théa Pharma GmbH, TRB-Chemedica AG, Ursapharm, and Visufarma, and a consultant for: Alcon, Alfa Intes, DMG, Dompé, Kala, Novaliq, Novartis, Santen GmbH, Senju, Shire, Sifi, Sun, Théa Pharma GmbH, TRB-Chemedica AG, Viatris, and Visufarma. Selina McGee, Jared Peterson, Assem Patel, Guruprasad Pattar, David G. Evans, and Gina Wesley have nothing to disclose. Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization guidelines, the principals of Good Clinical Practice, and applicable local regulatory requirements. The study protocol, informed consent form, and other information provided to participants, and all appropriate amendments were properly reviewed and approved by an Institutional Review Board (Alpha IRB, 1001 Avenida Pico, San Clemente, CA 92673).
Figures


References
-
- Boyd K. Redness-Relieving Eye Drops. American Academy of Opthalmology. 2023. https://www.aao.org/eye-health/treatments/redness-relieving-eye-drops.
-
- Corboz MR, Rivelli MA, Varty L, Mutter J, Cartwright M, Rizzo CA, et al. Pharmacological characterization of postjunctional alpha-adrenoceptors in human nasal mucosa. Am J Rhinol. 2005;19(5):495–502. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

