Durable effectiveness and safety of hybrid ablation versus catheter ablation: 2-year results from the randomized CEASE-AF trial†
- PMID: 40711852
- PMCID: PMC12304665
- DOI: 10.1093/ejcts/ezaf146
Durable effectiveness and safety of hybrid ablation versus catheter ablation: 2-year results from the randomized CEASE-AF trial†
Abstract
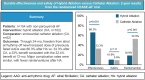
Objectives: The CEASE-AF trial demonstrated that epicardial-endocardial hybrid ablation (HA) had superior effectiveness compared to endocardial catheter ablation (CA) for non-paroxysmal atrial fibrillation (AF), without significantly increasing major complications during a 12-month period. Most contemporary AF ablation trials have not evaluated durability beyond 12 months. Therefore, 24-month effectiveness and safety of HA and CA are compared.
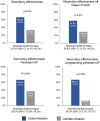
Methods: CEASE-AF is a prospective, multicentre, randomized trial. Patients 18-75 years of age with symptomatic, drug refractory persistent AF and left atrial diameter >4.0 cm or long-standing persistent AF were randomized 2:1 to HA (posterior wall and pulmonary vein isolation with left atrial appendage exclusion) or CA (pulmonary vein isolation). Secondary effectiveness was freedom from AF/atrial flutter/atrial tachycardia off class I/III anti-arrhythmic drugs except for those who previously failed at doses not exceeding those previously failed through a 24-month follow-up period. Major complications and reinterventions were evaluated.
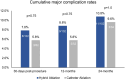
Results: The intention-to-treat population was 102 patients with HA and 52 patients with CA. Seventy-five percent were male, 80.5% had persistent AF and 19.5% had long-standing persistent AF, with a mean age of 60.7 ± 7.9 years. Effectiveness for 24 months was 66.3% (63/95) with HA and 33.3% (17/51) with CA [absolute difference 33.0% (95% confidence interval 14.3%, 48.3%; P < 0.001)]. Major complication rates were 10.8% (11/102) with HA and 9.6% (5/52) with CA (P = 1.0), and fewer patients had reinterventions after HA than CA [18.9% (18/95) vs 52.9% (27/51), P < 0.001].
Conclusions: CEASE-AF demonstrated that the 32.4% absolute benefit of HA over CA for 12 months was durable for 24 months at 33% with continued similar safety rates and fewer reinterventions after HA (funded by AtriCure, Inc.; NCT02695277).
Clinicaltrials.gov registration: NCT02695277.
Keywords: Atrial fibrillation; Catheter ablation; Hybrid ablation; Left atrial appendage; Surgical ablation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery.
Figures

References
-
- Kirchhof P, Camm AJ, Goette A et al. ; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. - PubMed
-
- Reddy VY, Gerstenfeld EP, Natale A et al. ; ADVENT Investigators. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–71. - PubMed
-
- Kuck KH, Brugada J, Furnkranz A et al. ; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. - PubMed
-
- Duytschaever M, De Potter T, Grimaldi M et al. ; inspIRE Trial Investigators. Paroxysmal atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circ Arrhythm Electrophysiol 2023;16:e011780. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

