Epicutaneous Staphylococcus aureus initiates cross-tissue IL-36R signaling for neutrophilic lung inflammation in a model of the atopic march
- PMID: 40711876
- PMCID: PMC12461889
- DOI: 10.1016/j.celrep.2025.116054
Epicutaneous Staphylococcus aureus initiates cross-tissue IL-36R signaling for neutrophilic lung inflammation in a model of the atopic march
Abstract
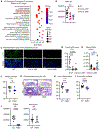
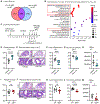
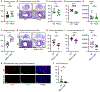
Patients with atopic dermatitis exhibit abundant Staphylococcus aureus skin colonization and an increased risk of atopic march diseases, including allergic rhinitis, food allergies, and asthma. We have previously shown that S. aureus skin exposure exacerbates allergic lung inflammation in an interleukin-36 receptor (IL-36R)-dependent manner. However, the cellular and molecular mechanisms by which S. aureus skin exposure and IL-36R signaling orchestrate the progression from skin to lung inflammation are unclear. Using a preclinical model of the atopic march, we found that S. aureus skin exposure promoted robust neutrophilic lung inflammation via keratinocyte- and lung epithelia-specific IL-36R signaling. Unexpectedly, neutrophil IL-36R signaling triggered neutrophil extracellular trap (NET) formation and augmented lung pathology. Importantly, anti-IL-36R monoclonal antibody (mAb) treatment prevented the development of neutrophilic lung inflammation. Collectively, our findings suggested that S. aureus skin exposure exacerbates lung inflammation via distinct IL-36R signaling mechanisms on epithelia and neutrophils, which has therapeutic potential in halting the progression of the atopic march.
Keywords: CP: Immunology; CP: Microbiology; IL-36; NETs; Staphylococcus aureus; atopic dermatitis; atopic march; immunotherapeutic; inflammation; lung; neutrophilic asthma; skin.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests N.K.A. has received previous grant support from Pfizer and Boehringer Ingelheim and was a paid consultant for Janssen Pharmaceuticals and Alphyn Biologics.
Figures



References
-
- Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, Margolis DJ, de Bruin-Weller M, and Eckert L (2018). Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 73, 1284–1293. - PubMed
-
- Weidinger S, Beck LA, Bieber T, Kabashima K, and Irvine AD (2018). Atopic dermatitis. Nat. Rev. Dis. Primers 4, 1–z. - PubMed
-
- Langan SM, Irvine AD, and Weidinger S (2020). Atopic dermatitis. Lancet 396, 345–360. - PubMed
-
- Hulpusch C, Weins AB, Traidl-Hoffmann C, and Reiger M (2021). A new era of atopic eczema research: Advances and highlights. Allergy 76, 3408–3421. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

