De-risking seizure liability: integrating adverse outcome pathways (AOPs), new approach methodologies (NAMs), and in silico approaches while highlighting knowledge gaps
- PMID: 40711969
- PMCID: PMC12469190
- DOI: 10.1093/toxsci/kfaf109
De-risking seizure liability: integrating adverse outcome pathways (AOPs), new approach methodologies (NAMs), and in silico approaches while highlighting knowledge gaps
Abstract
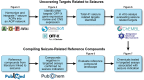
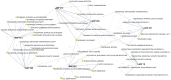
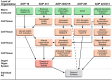
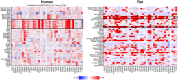
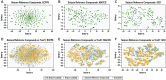
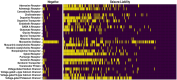
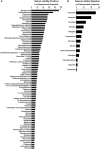
Animal studies are commonly used in drug development and in chemical and environmental toxicology to predict human toxicity, but their reliability, particularly in the central nervous system (CNS), is limited. For example, animal models often fail to predict drug-induced seizures, leading to unforeseen convulsions in clinical trials. Evaluating environmental compounds, such as pesticides, also poses challenges due to time and resource constraints, resulting in compounds remaining untested. To address these limitations, a government-industry collaboration identified 27 biological target families linked to seizure mechanisms by combining key events from adverse outcome pathways (AOPs) with drug discovery data. Over a hundred in vitro assay endpoints were identified, covering 26 of the target families, including neurotransmitter receptors, transporters, and voltage-gated calcium channels. A review of reference compounds identified 196 seizure-inducing and 34 seizure-negative chemicals, with 80% being tested in the in vitro assays. However, some target familes were more data-poor than others, highlighting significant data gaps. This proof-of-concept study demonstrates how mechanistic seizure liability can be assessed using an AOP framework and in vitro data. It underscores the need for expanded screening panels to include additional seizure-relevant targets. By integrating mechanistic insights into early drug development and environmental risk assessment, this approach enhances compound prioritization, complements animal studies, and optimizes resource use. Ultimately, this strategy refines CNS safety evaluation in drug development, improves public health protection to neurotoxicants, and bridges knowledge gaps.
Keywords: adverse outcome pathway (AOP); drug development; environmental exposure; in silico; new approach methodologies (NAMs); seizure.
Published by Oxford University Press on behalf of the Society of Toxicology 2025.
Figures
References
-
- Agency for Toxic Substances and Disease Registry (ATSDR). 2006. Toxicological profile for cyanide. Agency for Toxic Substances and Disease Registry (ATSDR) and U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [accessed 2025 Aug 2]. http://www.atsdr.cdc.gov/toxprofiles/tp8.html
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

