Recombinant human alpha-N-acetylglucosamine-6-sulfatase delivered to Sanfilippo D mice with repeated intracerebroventricular injections corrects CNS pathology
- PMID: 40711973
- PMCID: PMC12295227
- DOI: 10.1371/journal.pone.0328941
Recombinant human alpha-N-acetylglucosamine-6-sulfatase delivered to Sanfilippo D mice with repeated intracerebroventricular injections corrects CNS pathology
Abstract
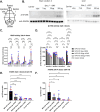
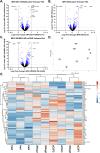
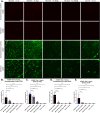
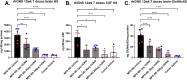
Mucopolysaccharidosis type IIID (MPS IIID; Sanfilippo D) is caused by biallelic pathogenic variants in N-acetylglucosamine-6-sulfatase (GNS), which participates in catabolism of heparan sulfate (HS) glycosaminoglycans. Characterization of MPS IIID disease at a cellular level has not been robustly achieved. We used unbiased quantitative proteomics to establish a cellular phenotype for MPS IIID mice. Recombinant human GNS (rhGNS), a variant of which previously demonstrated single dose efficacy in MPS IIID human fibroblasts and in MPS IIID neonatal mice, was used to establish a repeat dosing schedule to treat MPS IIID mice. Adult Gns KO mice or heterozygous carriers were treated via intracerebroventricular (ICV) injections and received 3, 30, or 200 μg rhGNS in 4 doses over 2 weeks or vehicle. Twenty-four hours after the final dose, HS in brain and CSF showed dose-dependent reductions, reaching carrier levels in the higher dose groups. Furthermore, the proteomic perturbations that we described were corrected by rhGNS treatment. Next, Gns KO or carrier adult mice were treated via ICV and received 3, 30 or 200 μg rhGNS or vehicle once every two weeks (Day 1, 15, 29, 43, 57, 71, 85) and were euthanized on day 91. Following treatment, total HS and MPS IIID-specific HS (GlcNAc6S) showed dose-dependent reductions in brain and CSF and markers of neuroinflammation were substantially reduced. ICV enzyme replacement therapy with rhGNS restores CNS pathology of adult MPS IIID mice even with treatment at 14-day intervals, demonstrating preclinical efficacy for MPS IIID.
Copyright: © 2025 Austin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
P.I.D. receives research support from Alnylam, M6P Therapeutics, and Biomarin Pharmaceutical Inc. J.W. and S.S. are employees of Phoenix Nest. P.I.D., T.F.C., and S.Q.L. are inventors on Patent #USSN 15/946,505; 0WVR-223143-US for Enzyme replacement therapy for mucopolysaccharidosis IIID. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

