Deep homology of a brachyury cis-regulatory syntax and the evolutionary origin of the notochord
- PMID: 40712008
- PMCID: PMC12292651
- DOI: 10.1126/sciadv.adw3307
Deep homology of a brachyury cis-regulatory syntax and the evolutionary origin of the notochord
Abstract
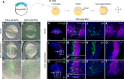
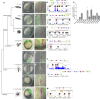
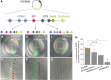
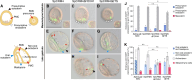
Expression of brachyury in the notochord is regarded as a chordate novelty and links to the origin of the notochord, yet the evolution of this regulatory control remains unclear. Here, we uncovered a regulatory syntax (named SFZE) consisting of binding sites for four transcription factors in notochord enhancers of chordate brachyury genes. SFZE was also identified in potential brachyury enhancers in various non-chordate animals and even in Capsaspora, a unicellular relative to animals. These non-chordate SFZE-containing enhancers exhibited activity in the zebrafish notochord. Furthermore, the SFZE syntax in a non-chordate confers endoderm activity. Our results indicate the ancient association of SFZE with brachyury, likely predating the origin of animals. The emergence of notochordal brachyury expression could be attributed to co-option of upstream signals acting on the conserved SFZE syntax, which facilitates the origin of the notochord from rudimentary endodermal cells.
Figures
References
-
- Stemple D. L., Structure and function of the notochord: An essential organ for chordate development. Development 132, 2503–2512 (2005). - PubMed
-
- Satoh N., Tagawa K., Lowe C. J., Yu J.-K., Kawashima T., Takahashi H., Ogasawara M., Kirschner M., Hisata K., Su Y.-H., Gerhart J., On a possible evolutionary link of the stomochord of hemichordates to pharyngeal organs of chordates: Stomochord and Notochord Relationship. Genesis 52, 925–934 (2014). - PMC - PubMed
-
- Lauri A., Brunet T., Handberg-Thorsager M., Fischer A. H. L., Simakov O., Steinmetz P. R. H., Tomer R., Keller P. J., Arendt D., Development of the annelid axochord: Insights into notochord evolution. Science 345, 1365–1368 (2014). - PubMed
-
- Hejnol A., Lowe C. J., Animal evolution: Stiff or squishy notochord origins? Curr. Biol. 24, R1131–R1133 (2014). - PubMed
-
- Bruce A. E. E., Winklbauer R., Brachyury in the gastrula of basal vertebrates. Mech. Dev. 163, 103625 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

