Structural analysis of HER2-trastuzumab complex reveals receptor conformational adaptation
- PMID: 40712014
- PMCID: PMC12292818
- DOI: 10.1126/sciadv.adu9945
Structural analysis of HER2-trastuzumab complex reveals receptor conformational adaptation
Abstract
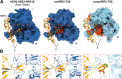
Human epidermal growth factor receptor-2 (HER2) is a receptor tyrosine kinase, associated with a variety of malignant tumors, usually through overexpression, resulting in aberrant signaling. Trastuzumab (TZB), one of the monoclonal antibodies (mAbs) used in combination with chemotherapy, has become a major therapeutic for HER2-overexpressing cancers. Current structural understanding of HER2 and its interactions with other receptors and with different affinity agents has relied on numerous structures of individual domains of HER2. Here, we subjected purified near full-length HER2 to single-particle cryo-electron microscopy (cryo-EM) analysis. Besides the canonical conformation described in previous structural studies, we report a previously unreported conformation of the HER2 extracellular domain that is stabilized upon TZB binding, which might hamper association with HER3, a receptor with which HER2 forms an oncogenic unit. Together, our findings provide insights into the conformational dynamics of the HER2 receptor and the mechanism of action of TZB.
Figures


References
-
- Yarden Y., Sliwkowski M. X., Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001). - PubMed
-
- Cho H. S., Mason K., Ramyar K. X., Stanley A. M., Gabelli S. B., Denney D. W. Jr., Leahy D. J., Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

