Thermoresponsive antioxidant metal-free carbon nanodot hydrogel: An effective therapeutic approach for ocular surface disease
- PMID: 40712015
- PMCID: PMC12292845
- DOI: 10.1126/sciadv.adt8775
Thermoresponsive antioxidant metal-free carbon nanodot hydrogel: An effective therapeutic approach for ocular surface disease
Abstract
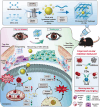
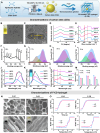
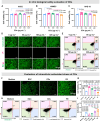
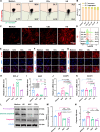
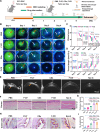
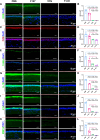
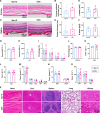
Oxidative stress, an imbalance between the body's oxidative and antioxidant systems, contributes markedly to the development of numerous ocular surface diseases, particularly dry eye disease (DED). Effective elimination or reduction of reactive oxygen species (ROS) can halt disease progression and alleviate symptoms. This study presents an innovative thermoresponsive, metal-free carbon nanodot (CD) hydrogel, termed F-CD hydrogel, which exhibits potent neutralization capabilities against multiple free radicals, including OH·, O2-·, and ·DPPH. This hydrogel demonstrates remarkable antioxidant, anti-inflammatory, and antiapoptotic capacity, effectively relieving DED symptoms by eliminating ROS at their source. Furthermore, its thermosensitive characteristics enhance the adherence of CDs to the ocular surface. In mouse models of DED, the F-CD hydrogel accelerates epithelial repair, thickens the corneal epithelium, promotes tear secretion, and boosts goblet cell count by up-regulating protective genes while suppressing those promoting apoptosis and oxidative stress. Coupled with its excellent biocompatibility and reduced administration frequency and treatment duration, the F-CD hydrogel emerges as a promising therapeutic approach for DED.
Figures


Similar articles
-
Astaxanthin ameliorates benzalkonium chloride-induced dry eye disease through suppressing inflammation and oxidative stress via Keap1-Nrf2/HO-1 signaling pathways.Animal Model Exp Med. 2025 Jun;8(6):1056-1079. doi: 10.1002/ame2.70000. Epub 2025 Mar 5. Animal Model Exp Med. 2025. PMID: 40045550 Free PMC article.
-
Medicinal Plant-Derived Carbon Dots Nanozymes Ameliorate Ulcerative Colitis via Anti-inflammatory, Antioxidant, and Gut Barrier-Protective Effects.ACS Appl Mater Interfaces. 2025 Jul 30;17(30):42751-42766. doi: 10.1021/acsami.5c08068. Epub 2025 Jul 3. ACS Appl Mater Interfaces. 2025. PMID: 40611517
-
Bovine amniotic membrane with antioxidant and anti-inflammatory properties for the repair of alkali-burned corneas.Acta Biomater. 2025 Jul 1;201:198-211. doi: 10.1016/j.actbio.2025.05.054. Epub 2025 May 23. Acta Biomater. 2025. PMID: 40414263
-
Topical corticosteroids for dry eye.Cochrane Database Syst Rev. 2022 Oct 21;10(10):CD015070. doi: 10.1002/14651858.CD015070.pub2. Cochrane Database Syst Rev. 2022. PMID: 36269562 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Stapleton F., Alves M., Bunya V. Y., Jalbert I., Lekhanont K., Malet F., Na K. S., Schaumberg D., Uchino M., Vehof J., Viso E., Vitale S., Jones L., TFOS DEWS II epidemiology report. Ocul. Surf. 15, 334–365 (2017). - PubMed
-
- Han K., Ju M. J., Kim D. H., Choi Y. H., Environmental exposures to lead, cadmium, and mercury and pterygium in Korean adults. Environ. Sci. Pollut. Res. Int. 29, 55058–55068 (2022). - PubMed
-
- Zicari A. M., Brindisi G., Castro G. D., Lollobrigida V., Nebbioso M., Duse M., Is oxidative stress involved in vernal keratoconjunctivitis? Results from a pilot study in children. Pediatr. Allergy Immunol. 31, 52–56 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

