Human iPSC-based breast cancer model identifies S100P-dependent cancer stemness induced by BRCA1 mutation
- PMID: 40712032
- PMCID: PMC12292934
- DOI: 10.1126/sciadv.adi2370
Human iPSC-based breast cancer model identifies S100P-dependent cancer stemness induced by BRCA1 mutation
Abstract
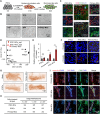
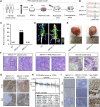
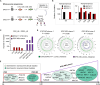
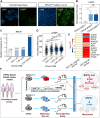
Breast cancer is the most common malignancy in females and remains the leading cause of cancer-related deaths for women worldwide. The cellular and molecular basis of breast tumorigenesis is not completely understood partly due to the lack of human research models which simulate the development of breast cancer. Here, we developed a method for generating functional mammary-like cells (MCs) from human-induced pluripotent stem cells (iPSCs). The iPSC-MCs closely resemble human primary MCs at cellular, transcriptional, and functional levels. Using this method, a breast cancer model was generated using patient-derived iPSCs harboring germline BRCA1 mutation. The patient iPSC-MCs recapitulated the transcriptome, clinical genomic alteration, and tumorigenic ability of breast cancer cells. We also identified S100P as an oncogene downstream of mutated BRCA1 that promotes cancer cell stemness and tumorigenesis. Our study establishes a promising system of breast cancer for studying the mechanism of tumorigenesis and identifying potential therapeutic targets.
Figures



References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). - PubMed
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

