Synergically enhanced anti-tumor immunity of in vivo panCAR by circRNA vaccine boosting
- PMID: 40712575
- PMCID: PMC12432353
- DOI: 10.1016/j.xcrm.2025.102250
Synergically enhanced anti-tumor immunity of in vivo panCAR by circRNA vaccine boosting
Abstract
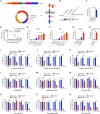
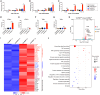
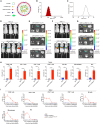
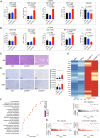
Chimeric antigen receptor (CAR) T cell therapy has shown promise in treating hematologic malignancies, but it still faces challenges, including high costs, a time-consuming manufacturing process, and the necessity of lymphodepletion. Here, we generate circular RNAs (circRNAs) encoding CAR proteins, referred to as circRNACAR, which mediates remarkable tumor killing in human primary T cells. We demonstrate that circRNACAR, delivered with immunocyte-tropic lipid nanoparticles (LNPs), can form in vivo panCAR cells (CAR-T, CAR-natural killer [NK], and CAR-macrophage), significantly inhibit tumor growth, and reshape the tumor microenvironment in mice. Importantly, combining in vivo panCAR with circRNA-based vaccines encoding the corresponding HER2 antigens exhibits synergistically enhanced anti-tumor immunity. Notably, circRNACAR can in return boost the level of vaccination-elicited HER2-specific antibodies, mediating effective killing of tumor cells by macrophages. In combination with vaccination, in vivo panCAR demonstrates a synergistic enhancement of anti-tumor immunity across various mouse models, thereby establishing a framework for the synergistic in vivo panCAR-VAC immunotherapy.
Keywords: RNA therapeutics; circRNA vaccine; in vivo CAR; off-the-shelf CAR; panCAR immunotherapy.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Patents related to the data presented in this manuscript have been filed.
Figures



References
-
- Amini L., Silbert S.K., Maude S.L., Nastoupil L.J., Ramos C.A., Brentjens R.J., Sauter C.S., Shah N.N., Abou-El-Enein M. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 2022;19:342–355. doi: 10.1038/s41571-022-00607-3. - DOI - PubMed
-
- Maalej K.M., Merhi M., Inchakalody V.P., Mestiri S., Alam M., Maccalli C., Cherif H., Uddin S., Steinhoff M., Marincola F.M., Dermime S. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol. Cancer. 2023;22:20. doi: 10.1186/s12943-023-01723-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

