Beneficial value of [18F]FDG PET/CT in the follow-up of patients with stage III non-small cell lung cancer (NVALT31-PET study): study protocol of a multicentre randomised controlled trial
- PMID: 40713037
- PMCID: PMC12306341
- DOI: 10.1136/bmjopen-2025-103745
Beneficial value of [18F]FDG PET/CT in the follow-up of patients with stage III non-small cell lung cancer (NVALT31-PET study): study protocol of a multicentre randomised controlled trial
Abstract
Introduction: Patients with stage III non-small cell lung cancer (NSCLC) are at high risk of developing post-treatment recurrences (50-78%) during follow-up. As more effective treatments are now available, especially for patients with oligometastatic disease, earlier detection of recurrences may prolong survival and health-related quality of life (HRQOL). With the use of 2'-deoxy-2'-[18F]fluoroglucose positron emission tomography/CT ([18F]FDG PET/CT) during follow-up, recurrences may be detected earlier. Therefore, the primary objective of this study is to compare the 3-year overall survival of patients with stage III NSCLC during follow-up surveillance with [18F]FDG PET/CT versus follow-up with conventional CT (usual care). Secondary objectives address the number, location and timing of recurrences, as well as HRQOL, cost-effectiveness and patient experiences of PET/CT scans.
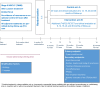
Methods and analysis: In this multicentre randomised controlled clinical trial, 690 patients with stage III NSCLC (8th edition International Association for the Study of Lung Cancer (IASLC) Tumor, Nodes, Metastasis (TNM) classification) who completed curative intended treatment and started follow-up care (which may include adjuvant therapy) will be randomised 1:1 to either the intervention ([18F]FDG PET/CT) or the control group (CT). Patients will undergo follow-up scans during visits at 6, 12, 18, 24 and 36 months. Data will be collected using validated questionnaires, electronic case report forms and data extractions from the electronic health records. Additionally, blood samples will be collected, and interviews will be conducted.
Ethics and dissemination: The study protocol has been approved by the Medical Ethical Committee of the Radboudumc and review boards of all participating centres. Written informed consent will be obtained from all participants. Study results will be published in international peer-reviewed scientific journals and presented at relevant scientific conferences. Data will be published in a data repository or other online data archive.
Trial registration number: NCT06082492.
Keywords: Diagnostic Imaging; ONCOLOGY; Respiratory tract tumours.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SS: Varian Medical Systems – Departmental research support. AstraZeneca – Institutional research support, speaker’s honoraria, trial steering committee (payments to institution). MSD – Speaker’s honoraria, consultancy (payments to institution). Lilly – Steering committee (payments to institution). MvdH: MvdH has received institutional grant funding and payments for educational activities/webinars. MvdH is a member of advisory boards for AbbVie, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Roche, Sanofi and Takeda. MvdH is chair of the NVALT Studies Foundation and chair of the oncology section of NVALT. MvdH serves as local principal investigator for clinical trials sponsored by AstraZeneca, BMS, GSK, Novartis, Merck, Roche, Takeda, Mirati, AbbVie, MSD, Merck, Amgen, Boehringer Ingelheim and Pfizer. All payments were made to the institution. All other authors have no competing interests to declare.
Figures
Similar articles
-
The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation.Health Technol Assess. 2011 Sep;15(35):1-192, iii-iv. doi: 10.3310/hta15350. Health Technol Assess. 2011. PMID: 21958472 Free PMC article.
-
PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer.Cochrane Database Syst Rev. 2014 Nov 13;2014(11):CD009519. doi: 10.1002/14651858.CD009519.pub2. Cochrane Database Syst Rev. 2014. PMID: 25393718 Free PMC article.
-
Surveillance With Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography of Patients With Stage I-to-III Lung Cancer After Completion of Curative treatment (SUPE_R): A Randomized Controlled Trial.J Thorac Oncol. 2025 Aug;20(8):1086-1097. doi: 10.1016/j.jtho.2025.04.003. Epub 2025 Apr 19. J Thorac Oncol. 2025. PMID: 40258572 Clinical Trial.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
References
-
- NVALT Richtlijn Niet-kleincellig longcarcinoom - Follow up NSCLC-patiënt na curatieve behandeling. 2020
-
- Stirling RG, Chau C, Shareh A, et al. Effect of Follow-Up Surveillance After Curative-Intent Treatment of NSCLC on Detection of New and Recurrent Disease, Retreatment, and Survival: A Systematic Review and Meta-Analysis. J Thorac Oncol. 2021;16:784–97. doi: 10.1016/j.jtho.2021.01.1622. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials