Master protocols in low- and middle-income countries: a review of current use, limitations and opportunities for precision medicine
- PMID: 40713083
- PMCID: PMC12306224
- DOI: 10.1136/bmjgh-2024-018561
Master protocols in low- and middle-income countries: a review of current use, limitations and opportunities for precision medicine
Abstract
Background: Master protocols-umbrella, basket and platform trials, study multiple therapies, multiple diseases or both, offering many advantages, most profoundly that they answer multiple treatment-related questions, which would otherwise take multiple trials. We conducted a review of clinical trial registries to characterise their use in advancing precision medicine in low and middle-income countries (LMICs).
Methods: We searched trial records available in 20 trial registries globally, including ClinicalTrials.gov and WHO ICTRP, to identify umbrella, basket and platform trials launched until 30 September 2023.
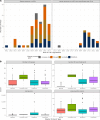
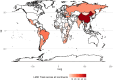
Results: We identified 102 master protocols-29 umbrella trials, 31 basket trials, 36 platform trials, as well as six other designs that partially aligned with the working definition of master protocols, run in 54 different LMICs. Most trials were pharmaceutical industry-sponsored studies (60/102, 58.8%), conducted in oncology settings (56/102, 54.9%), currently ongoing (69/102, 67.6%) in early phase (phase I and II) settings (70/102, 68.6%) and have been planned or launched in the last 5 years (93/102, 91.2%), mainly with international collaborations in high-income countries. China was a site to more than half of all master protocols (53/102, 52%), and only a small proportion of trials (5/102, 4.9%) launched exclusively in LMICs excluding China and European middle-income countries. For most studies, aspects of trial design and trial documentation (including study protocols and analysis plans) were not publicly accessible.
Conclusion: Unlike high-income countries, where several hundreds of master protocols are ongoing or completed, there is limited use of master protocols in LMICs, partly owing to low penetration of precision medicine research and limited clinical trial infrastructure in most LMICs. The evidence presented here creates a case for supporting precision medicine initiatives in LMICs (especially Africa) and training and capacity building initiatives focused on innovative clinical trial designs like master protocols, especially in therapeutic areas outside oncology.
Keywords: Clinical trial; Global Health; Randomised control trial; Review.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Methods for blood loss estimation after vaginal birth.Cochrane Database Syst Rev. 2018 Sep 13;9(9):CD010980. doi: 10.1002/14651858.CD010980.pub2. Cochrane Database Syst Rev. 2018. PMID: 30211952 Free PMC article.
-
Interventions to improve access to cataract surgical services and their impact on equity in low- and middle-income countries.Cochrane Database Syst Rev. 2017 Nov 9;11(11):CD011307. doi: 10.1002/14651858.CD011307.pub2. Cochrane Database Syst Rev. 2017. PMID: 29119547 Free PMC article.
-
Oxytocin for preventing postpartum haemorrhage (PPH) in non-facility birth settings.Cochrane Database Syst Rev. 2016 Apr 14;4(4):CD011491. doi: 10.1002/14651858.CD011491.pub2. Cochrane Database Syst Rev. 2016. PMID: 27078125 Free PMC article.
-
Specially formulated foods for treating children with moderate acute malnutrition in low- and middle-income countries.Cochrane Database Syst Rev. 2013 Jun 21;(6):CD009584. doi: 10.1002/14651858.CD009584.pub2. Cochrane Database Syst Rev. 2013. PMID: 23794237
-
Public stewardship of private for-profit healthcare providers in low- and middle-income countries.Cochrane Database Syst Rev. 2016 Aug 11;2016(8):CD009855. doi: 10.1002/14651858.CD009855.pub2. Cochrane Database Syst Rev. 2016. PMID: 27510030 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
