Impact of first-line tyrosine kinase inhibitor selection on survival outcomes with second-line nivolumab in metastatic renal cell carcinoma
- PMID: 40713516
- PMCID: PMC12296657
- DOI: 10.1186/s12885-025-14654-3
Impact of first-line tyrosine kinase inhibitor selection on survival outcomes with second-line nivolumab in metastatic renal cell carcinoma
Abstract
Introduction: Access to first-line immune checkpoint inhibitor (ICI) combinations in metastatic renal cell carcinoma (mRCC) remains limited in many low- and middle-income countries. Consequently, tyrosine kinase inhibitors (TKIs) are still widely used. This study investigates the impact of first-line sunitinib versus pazopanib on survival outcomes with second-line nivolumab.
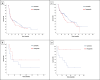
Methods: We conducted a retrospective analysis of 245 patients with mRCC from the Turkish Oncology Group Kidney Cancer Consortium Database. Patients received first-line sunitinib or pazopanib, followed by second-line nivolumab. Primary endpoints were time to treatment failure (TTF) and overall survival (OS). Subgroup analyses were performed based on IMDC risk classification and presence of sarcomatoid features.
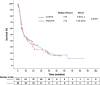
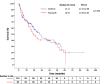
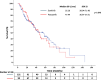
Results: A total of 245 patients who were treated with sunitinib or pazopanib monotherapy as first-line treatment followed by nivolumab as second-line treatment were included in this study. Median TTF following nivolumab initiation was similar between prior sunitinib and pazopanib groups (7.79 vs 7.72 months; p = 0.892). Median OS-2 was 27.21 months with prior sunitinib and 18.92 months with prior pazopanib (p = 0.496). In patients with sarcomatoid features (n = 20), those pretreated with pazopanib demonstrated numerically longer OS-2 compared to sunitinib (p = 0.023), although the small sample size limits definitive conclusions.
Conclusion: No significant differences in survival outcomes were observed between first-line sunitinib and pazopanib before nivolumab in mRCC. In the small subgroup with sarcomatoid features, pazopanib pre-treatment was associated with a numerically longer survival. These findings warrant cautious interpretation and further prospective validation, especially in resource-constrained settings.
Keywords: Metastatic renal cell cancer; Nivolumab; Pazopanib; Sunitinib.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ankara University Faculty Ethics Committee (date:11/09/2024, approval number I09-701–24) and it was carried out in accordance with the Code of Ethics of the World Medical Association also known as a declaration of Helsinki. Due to the retrospective nature of the study the use of anonymized data, no informed consent was required as per the ethics committee approval. Consent for publication: Not applicable. Competing interests: Yüksel Ürün: Advisory work —Abdi-İbrahim, Astellas, AstraZeneca, Bristol Myers-Squibb, Deva, Eczacıbaşı, Gen ilaç, Gilead, GSK, Janssen, Merck, MSD, Novartis, Pfizer, Roche; travel, honoraria and consultation fees—Abdi-İbrahim, Astellas, Bristol Myers-Squibb, Deva, Eczacıbaşı, Gen ilaç, Gilead, GSK, Janssen, Merck, Novartis, Pfizer, Roche. All remaining authors have declared no conflicts of interest.
Figures
References
-
- Bölek H, Sertesen E, Kuzu OF, Tural D, Sim S, Şendur MAN, et al. Treatment patterns and attrition in metastatic renal cell carcinoma: real-life experience from the Turkish Oncology Group Kidney Cancer Consortium (TKCC) database. Clin Genitourin Cancer. 2025;23(1):102282. - PubMed
-
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. - PubMed
-
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–20. - PubMed
-
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. - PubMed
-
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

