Extracellular vesicles from ovarian cancer cells induce senescent lipid-laden macrophages to facilitate omental metastasis
- PMID: 40713812
- PMCID: PMC12297738
- DOI: 10.1186/s12951-025-03612-7
Extracellular vesicles from ovarian cancer cells induce senescent lipid-laden macrophages to facilitate omental metastasis
Abstract
Background: Ovarian cancer exhibits striking metastatic tropism for the omentum, where lipid-laden macrophages are key mediators that fuel disease progression. However, the mechanisms governing their formation and pro-metastatic functions remain poorly understood. As extracellular vesicles (EVs) have as critical regulators of tumor-stroma crosstalk in metastatic niches, we sought to define how ovarian cancer-derived EVs orchestrate macrophages and adipocytes, and their impact on omental metastasis, aiming to explore potential therapeutic interventions.
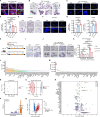
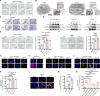
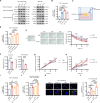
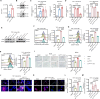
Results: Single-cell transcriptomics of ovarian cancer revealed a distinct lipid-laden macrophage population in omentum, whose abundance correlated with metastatic burden and poor survival. Proteomics revealed that EVs from highly metastatic ovarian cancer cells were enriched in lipid metabolism regulators. In vivo experiments demonstrated that these tumor-derived vesicles mediated macrophage reprogramming, driving the acquisition of a pro-metastatic phenotype. Quantitative lipidomic profiling and lipid staining approaches confirmed the progressive lipid-laden in EV-treated macrophages. Using a patient-derived omentum-macrophage co-culture system, we demonstrated that tumor-derived EVs stimulate lipid release from omental adipocytes, which macrophages subsequently internalize through CD36-dependent uptake to drive lipid accumulation. This metabolic reprogramming culminated in cellular senescence, as evidenced by classical biomarkers including SA-β-galactosidase activity, elevated p16-INK4A and p53 levels, and the development of a matrix metalloproteinase-enriched senescence-associated secretory phenotype. Immunohistochemistry of clinical specimens demonstrated overexpression of CD36 correlated with omental metastasis and poor survival in ovarian cancer. In vivo experiments demonstrated that CD36 inhibition and senolytic therapy attenuated omental metastasis.
Conclusions: This study unveils an EV-driven mechanism of adipose tropism in ovarian cancer metastasis, where EVs promote the formation of senescent lipid-laden macrophages via CD36-mediated lipid uptake, remodeling the metastatic niche. Targeting CD36 and senescent cells offers a promising therapeutic strategy against omental metastasis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All human specimens were obtained following the Declaration of Helsinki, and all participants provided informed written consent. The procedures were conducted following ethical guidelines and obtained approval from the Medical Ethics Committee, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Animals were handled according to the recommendation of the Regulations for the Administration of Affairs Concerning Experimental Animals of China. The procedures were approved by the Laboratory Animal Ethics Committee of Wuhan Youdu Biotechnology Ltd. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Adipocyte-derived factors induce adherent to suspension transition in breast and pancreatic cancer cells through lipid metabolic alteration.Sci Rep. 2025 Aug 20;15(1):30552. doi: 10.1038/s41598-025-13309-4. Sci Rep. 2025. PMID: 40836054 Free PMC article.
-
Omental Preadipocytes Stimulate Matrix Remodeling and IGF Signaling to Support Ovarian Cancer Metastasis.Cancer Res. 2024 Jul 2;84(13):2073-2089. doi: 10.1158/0008-5472.CAN-23-2613. Cancer Res. 2024. PMID: 38635891 Free PMC article.
-
The RNA binding protein IGF2BP2/IMP2 alters the cargo of cancer cell-derived extracellular vesicles supporting tumor-associated macrophages.Cell Commun Signal. 2024 Jun 27;22(1):344. doi: 10.1186/s12964-024-01701-y. Cell Commun Signal. 2024. PMID: 38937789 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
"Small extracellular vesicles: messengers at the service of breast cancer agenda in the primary and distant microenvironments".J Exp Clin Cancer Res. 2025 Jul 21;44(1):216. doi: 10.1186/s13046-025-03471-y. J Exp Clin Cancer Res. 2025. PMID: 40691627 Free PMC article. Review.
References
-
- Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. - PubMed
-
- Schoutrop E, Moyano-Galceran L, Lheureux S, Mattsson J, Lehti K, Dahlstrand H, Magalhaes I. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin Cancer Biol. 2022;86:207–23. - PubMed
-
- Timperi E, Gueguen P, Molgora M, Magagna I, Kieffer Y, Lopez-Lastra S, Sirven P, Baudrin LG, Baulande S, Nicolas A, et al. Lipid-Associated macrophages are induced by Cancer-Associated fibroblasts and mediate immune suppression in breast Cancer. Cancer Res. 2022;82:3291–306. - PubMed
-
- Papavassiliou KA, Papavassiliou AG. Hungry for fat: metabolic crosstalk with lipid-rich CAFs fuels pancreatic cancer. Cell Metab. 2024;36:1172–4. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

