Interleukin-6 and its association with outcome in traumatic brain injury: a prospective cohort
- PMID: 40713822
- PMCID: PMC12296603
- DOI: 10.1186/s13049-025-01430-2
Interleukin-6 and its association with outcome in traumatic brain injury: a prospective cohort
Abstract
Background: Traumatic brain injury (TBI) continues to be a major cause of death and disability worldwide. Biomarkers for treatment and prognostication are needed for counseling and clinical management.
Objective: In this study, we evaluated the ability of serum IL-6 to predict mortality and disability in a population whith moderate and severe TBI (msTBI).
Methods: Adult patients with msTBI were included consecutively from December 2019 to August 2023. Clinical data were collected during hospital stays and functional outcome was established at 6 months using GOSE. Serum IL-6 levels were measured on day 0, day 3 and day 7 after injury.
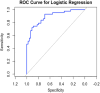
Results: Eighty-eight patients were recruited and completed 6-month follow-up. Clinical variables associated with the 6-month adverse outcome were admission GCS (OR 0.77 95% CI 0.67-0.87, p < 0.001), age (OR 1.10 95% CI 1.03-1.1, p = 0.001), Rotterdam score (OR 2.8 95% CI 1.7-5.0, p < 0.001), hospital infections (OR 4.7 95% CI 1.9-12.1, p < 0.001) and day-0 IL-6 (OR 1.1 95% CI 1.08-1.13, p < 0.001). When adjusted for age, severity of injury,and the presence of a hospital infection, day-0 IL-6 was significantly associated with the adverse outcome at 6 months (OR 1.15 95% CI 1.1-1.2, p = 0.031). Area under the curve (AUC) of 89% (95% CI 82%-96%). Calculated sensitivity and specificity were 75% and 89%, respectively, at a cut-off point of 59 pg/ml.
Conclusion: In a population of msTBI, levels of serum interleukin-6 within the first 24 h after injury is an independent predictor of 6-month mortality and disability with a net benefit in clinical decision-making across relevant threshold probabilities.
Keywords: Biomarker; Inflammation; Inflammatory response; Interleukin; Prognostication; Trauma; Traumatic brain injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Blood biomarkers for the non-invasive diagnosis of endometriosis.Cochrane Database Syst Rev. 2016 May 1;2016(5):CD012179. doi: 10.1002/14651858.CD012179. Cochrane Database Syst Rev. 2016. PMID: 27132058 Free PMC article.
-
Parallel Cerebrospinal Fluid and Serum Temporal Profile Assessment of Axonal Injury Biomarkers Neurofilament-Light Chain and Phosphorylated Neurofilament-Heavy Chain: Associations With Patient Outcome in Moderate-Severe Traumatic Brain Injury.J Neurotrauma. 2024 Jul;41(13-14):1609-1627. doi: 10.1089/neu.2023.0449. Epub 2024 May 13. J Neurotrauma. 2024. PMID: 38588256 Free PMC article.
-
Does the Presence of Missing Data Affect the Performance of the SORG Machine-learning Algorithm for Patients With Spinal Metastasis? Development of an Internet Application Algorithm.Clin Orthop Relat Res. 2024 Jan 1;482(1):143-157. doi: 10.1097/CORR.0000000000002706. Epub 2023 Jun 12. Clin Orthop Relat Res. 2024. PMID: 37306629 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Elevation of the head during intensive care management in people with severe traumatic brain injury.Cochrane Database Syst Rev. 2017 Dec 28;12(12):CD009986. doi: 10.1002/14651858.CD009986.pub2. Cochrane Database Syst Rev. 2017. PMID: 29283434 Free PMC article.
References
-
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. 10.1016/S1474-4422(18)30415-0 Epub 2018 Nov 26. Erratum in: Lancet Neurol. 2021 Dec;20(12):e7. https://doi.org/10.1016/S1474-4422(21)00383-5 . PMID: 30497965; PMCID: PMC6291456 . - PMC - PubMed
-
- Mccrea MA, Giacino JT, Barber J, Temkin NR, Nelson LD, Levin HS, Dikmen S, Stein M, Bodien YG, Boase K, Taylor SR, Vassar M, Mukherjee P, Robertson C, Diaz-Arrastia R, Okonkwo DO, Markowitz AJ, Manley GT, TRACK-TBI Investigators, Adeoye O, Badjatia N, Bullock MR, Chesnut R, Corrigan JD, Crawford K, Duhaime AC, Ellenbogen R, Feeser VR, Ferguson AR, Foreman B, Gardner R, Gaudette E, Goldman D, Gonzalez L, Gopinath S, Gullapalli R, Hemphill JC, Hotz G, Jain S, Keene CD, Korley FK, Kramer J, Kreitzer N, Lindsell C, Machamer J, Madden C, Martin A, McAllister T, Merchant R, Ngwenya LB, Noel F, Nolan A, Palacios E, Perl D, Puccio A, Rabinowitz M, Rosand J, Sander A, Satris G, Schnyer D, Seabury S, Sherer M, Toga A, Valadka A, Wang K, Yue JK, Yuh E, Zafonte R. Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study. JAMA Neurol. 2021;78(8):982–92. 10.1001/jamaneurol.2021.2043. PMID: 34228047; PMCID: PMC8261688 - PMC - PubMed
-
- Andelic N, Howe EI, Hellstrøm T, Sanchez MF, Lu J, Løvstad M, Røe C. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. 10.1002/brb3.1018 Epub 2018 Jun 11. Erratum in: Brain Behav. 2021 Aug;11(8):e02120. https://doi.org/10.1002/brb3.2120 . PMID: 29888869; PMCID: PMC6043714 . - PMC - PubMed
-
- Maas AIR, Menon DK, Manley GT, Abrams M, Åkerlund C, Andelic N, Aries M, Bashford T, Bell MJ, Bodien YG, Brett BL, Büki A, Chesnut RM, Citerio G, Clark D, Clasby B, Cooper DJ, Czeiter E, Czosnyka M, Dams-O’Connor K, De Keyser V, Diaz-Arrastia R, Ercole A, van Essen TA, Falvey É, Ferguson AR, Figaji A, Fitzgerald M, Foreman B, Gantner D, Gao G, Giacino J, Gravesteijn B, Guiza F, Gupta D, Gurnell M, Haagsma JA, Hammond FM, Hawryluk G, Hutchinson P, van der Jagt M, Jain S, Jain S, Jiang JY, Kent H, Kolias A, Kompanje EJO, Lecky F, Lingsma HF, Maegele M, Majdan M, Markowitz A, McCrea M, Meyfroidt G, Mikolić A, Mondello S, Mukherjee P, Nelson D, Nelson LD, Newcombe V, Okonkwo D, Orešič M, Peul W, Pisică D, Polinder S, Ponsford J, Puybasset L, Raj R, Robba C, Røe C, Rosand J, Schueler P, Sharp DJ, Smielewski P, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Temkin N, Tenovuo O, Theadom A, Thomas I, Espin AT, Turgeon AF, Unterberg A, Van Praag D, van Veen E, Verheyden J, Vyvere TV, Wang KKW, Wiegers EJA, Williams WH, Wilson L, Wisniewski SR, Younsi A, Yue JK, Yuh EL, Zeiler FA, Zeldovich M, Zemek R, InTBIR Participants and Investigators. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21(11):1004–60. 10.1016/S1474-4422(22)00309-X Epub 2022 Sep 29. Erratum in: Lancet Neurol. 2022 Dec;21(12):e10. https://doi.org/10.1016/S1474-4422(22)00411-2 . PMID: 36183712; PMCID: PMC10427240 . - PMC - PubMed
-
- Maas AIR, Hemphill JC, Wilson L, Manley GT. Managing outcome expectations after Traumatic Brain Injury. Injury. 2023;54(5):1233–5. 10.1016/j.injury.2023.03.027. PMID: 37055145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

