Impact of the Prosigna assay on neoadjuvant treatment decision-making in patients with early-stage HR-positive/HER2-negative breast cancer: a single-center prospective observational study
- PMID: 40714511
- PMCID: PMC12314373
- DOI: 10.1016/j.esmoop.2025.105521
Impact of the Prosigna assay on neoadjuvant treatment decision-making in patients with early-stage HR-positive/HER2-negative breast cancer: a single-center prospective observational study
Abstract
Background: The 50-gene assay Prosigna is cleared by the United States Food and Drug Administration only in postoperative hormone receptor-positive (HR-positive) breast cancer (BC) specimens. Given studies showing that Prosigna may identify chemo-sensitive tumors, this decision-impact study evaluated whether the assay could influence physicians' choices and patients' confidence in the neoadjuvant treatment plan.
Patients and methods: This single-center prospective observational study included patients with early-stage HR-positive/HER2-negative BC measuring ≥0.5 cm, any nodal status, deemed as possible candidates for neoadjuvant treatment per physician's choice. Formalin-fixed, paraffin-embedded core biopsy specimens were centrally analyzed using Prosigna. Physicians and patients were surveyed before and after Prosigna testing. The primary endpoint was the proportion of patients whose treatment differed from the pre-Prosigna recommendation. Secondary endpoints included assessing (i) reasons for treatment changes, (ii) physicians' and patients' confidence in the treatment recommendation and (iii) the association of residual cancer burden (RCB) with risk of recurrence (ROR) risk groups (RG).
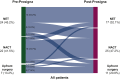
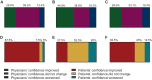
Results: Fifty-four patients were enrolled between March 2019 and April 2023. Tumors were luminal A, luminal B, HER2-enriched and basal-like in 27.8%, 63.0%, 5.6% and 3.7% of cases, respectively. ROR RG was low, intermediate, and high in 18.5%, 20.4% and 61.1% of cases, respectively. A change in the treatment plan occurred in 35.2% of cases [95% confidence interval (CI) 23.1%-50.2%]. Among 14 questionnaires regarding reasons for treatment changes, ROR RG alone and both ROR RG and intrinsic subtype were reported in 35.7% and 64.3% of cases, respectively. Of treating physicians, 83.7% felt their confidence in the appropriateness of the treatment plan remained stable or improved, and 62.5% of patients reported reduced anxiety. No association between RCB and ROR RG was found.
Conclusions: Performing Prosigna on upfront core biopsies may influence the neoadjuvant treatment decision-making in early-stage HR-positive/HER2-negative BC.
Clinicaltrials: gov number, NCT03749421.
Keywords: Prosigna; breast cancer; decision support; neoadjuvant; precision medicine.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2015 Apr 7;(4):CD010260. doi: 10.1002/14651858.CD010260.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Aug 22;8:CD010260. doi: 10.1002/14651858.CD010260.pub3. PMID: 25847525 Updated.
-
Predictive Value of Excision Repair Cross Complementation Group 1 (ERCC1) by Immunohistochemistry for Determining Neoadjuvant Chemotherapy Response in Triple-Negative Breast Cancers.Breast J. 2025 Feb 18;2025:8410670. doi: 10.1155/tbj/8410670. eCollection 2025. Breast J. 2025. PMID: 40008380 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Differences in the effectiveness of individual-level smoking cessation interventions by socioeconomic status.Cochrane Database Syst Rev. 2025 Jan 27;1(1):CD015120. doi: 10.1002/14651858.CD015120.pub2. Cochrane Database Syst Rev. 2025. PMID: 39868569
References
-
- Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
-
- Loibl S., André F., Bachelot T., et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35(2):159–182. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R., Davies C., Godwin J., et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

