IL-33-primed NLRP3 inflammasome in basophils drives IL-1β production and initiates atopic dermatitis inflammation
- PMID: 40715049
- PMCID: PMC12297455
- DOI: 10.1038/s41420-025-02630-6
IL-33-primed NLRP3 inflammasome in basophils drives IL-1β production and initiates atopic dermatitis inflammation
Abstract
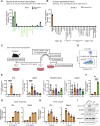
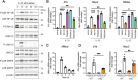
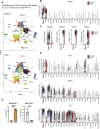
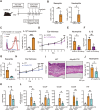
Atopic dermatitis (AD) is a chronic inflammatory skin disorder caused by immune dysregulation that involves the release of various pro-inflammatory cytokines. Patients with AD frequently exhibit basophil infiltration in the affected skin. Although the role of the NLRP3 inflammasome in innate immune cells has been extensively studied, the contribution of the basophil inflammasome to the pathophysiology of AD remains to be elucidated. In this study, we demonstrated that IL-33 primes the NLRP3 inflammasome in basophils, leading to the production and release of mature IL-1β. Mechanistically, we showed that IL-33 stimulation induced pro-IL-1β and NLRP3 expression via the NF-κB and p38 MAPK pathways and that basophils released mature IL-1β through the canonical inflammasome activation pathway, which requires NLRP3, ASC, caspase-1, and gasdermin D (GSDMD). In an oxazolone (OXA)-induced AD mouse model, we found that basophils acted as key initiators of inflammation by producing IL-1β in the lesion, and that basophil depletion, genetic ablation of Nlrp3 or Il1b, or basophil-specific genetic ablation of Nlrp3 ameliorated ear swelling and neutrophil infiltration. Collectively, these findings establish basophils as a significant early source of NLRP3 inflammasome-driven IL-1β, contributing to the pathogenesis of AD. Targeting the IL-33/ST2L axis or NLRP3 inflammasome activation in basophils may offer a promising therapeutic strategy for managing AD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal experiments were approved by the Use and Care of Experimental Animals Committee of Jichi Medical University (permission number 20116-02) and were carried out following Jichi Medical University guidelines.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

