Flower dependent trafficking of lamellar bodies facilitates maturation of the epidermal barrier
- PMID: 40715079
- PMCID: PMC12297442
- DOI: 10.1038/s41467-025-62105-1
Flower dependent trafficking of lamellar bodies facilitates maturation of the epidermal barrier
Abstract
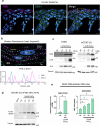
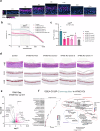
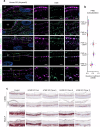
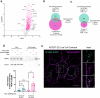
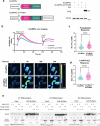
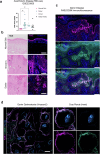
Specialized secretory cells, including keratinocytes in the last viable layers of mammalian epidermis, utilize lysosome-related organelles (LROs) to exocytose distinct cargoes vital for tissue function. Here, we demonstrate that the Flower isoform, hFWE4, a putative Ca2+ channel that permits endocytic retrieval of presynaptic vesicles and lytic granules, also resides on epidermal lamellar bodies (LBs), an LRO that extrudes a proteinaceous lipid-rich matrix to finalize the epidermal barrier. In differentiated keratinocyte cultures, we show that hFWE4-positive LB-like vesicles associate with a distinct ensemble of LRO trafficking mediators and demonstrate that hFWE4 liberates Ca2+ from intracellular stores to enable the surface presentation of cargo contained within these vesicles. Finally, supporting a critical role for hFWE4-dependent trafficking in establishing the epidermal barrier, we demonstrate that this process is dysregulated in genetic diseases of cornification that are driven by impairments in keratinocyte Ca2+ handling. Our results provide new insight into the biogenesis and trafficking of epidermal LBs and more broadly suggest that hFWE4 may serve as a core component of LRO trafficking machinery that endows Ca2+ dependency to distinct stages of the transport process depending on the cell of origin.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Candi, E., Schmidt, R. & Melino, G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol.6, 328–340 (2005). - PubMed
-
- Rassner, U., Feingold, K. R., Crumrine, D. A. & Elias, P. M. Coordinate assembly of lipids and enzyme proteins into epidermal lamellar bodies. Tissue Cell31, 489–498 (1999). - PubMed
-
- Oren, A., Ganz, T., Liu, L. & Meerloo, T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp. Mol. Pathol.74, 180–182 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

