Ferroptosis mediated by the IDO1/Kyn/AhR pathway triggers acute thymic involution in sepsis
- PMID: 40715082
- PMCID: PMC12297531
- DOI: 10.1038/s41419-025-07882-9
Ferroptosis mediated by the IDO1/Kyn/AhR pathway triggers acute thymic involution in sepsis
Abstract
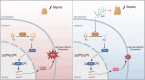
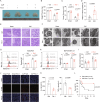
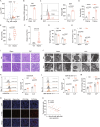
Acute thymic involution (ATI) is frequently observed during sepsis, however the underlying mechanisms remain poorly understood. This study demonstrates that ferroptosis plays a crucial role in sepsis-associated ATI. We found that pediatric sepsis patients showed significantly elevated kynurenine (Kyn)/tryptophan (Trp) ratios, indicating increased indoleamine 2,3-dioxygenase 1 (IDO1) activity, along with higher Kyn levels compared to controls. Moreover, Kyn levels were negatively correlated with thymus-to-thorax ratio. Further mechanistic analysis revealed that the enhanced expression of IDO1, induced by inflammatory signals, drives the accumulation of Kyn and subsequent activation of the aryl hydrocarbon receptor (AhR), triggering lipid oxidation-related gene transcription and ferroptosis in thymocytes during sepsis. Treatment with 1-methyltryptophan (IDO1 inhibitor) effectively restore thymic function and improve survival in septic mice. Our findings reveal a novel role for the IDO1/Kyn/AhR pathway in ferroptosis, suggesting that targeting this pathway may offer a promising therapeutic strategy for sepsis. Created with BioRender ( https://app.biorender.com/ ).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Abraham E. New definitions for sepsis and septic shock: continuing evolution but with much still to be done. JAMA. 2016;315:757–9. - PubMed
-
- van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–20. - PubMed
-
- Boehm T, Swann JB. Thymus involution and regeneration: two sides of the same coin?. Nat Rev Immunol. 2013;13:831–8. - PubMed
-
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

