Entropy-driven denaturation enables sustainable protein regeneration through rapid gel-solid transition
- PMID: 40715083
- PMCID: PMC12297245
- DOI: 10.1038/s41467-025-61959-9
Entropy-driven denaturation enables sustainable protein regeneration through rapid gel-solid transition
Abstract
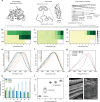
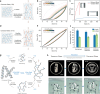
The upcycling of protein materials has long been hindered by the difficulty in restructuring them to usable forms. In contrast to proteins extracted using conventional organic denaturants, keratin treated with concentrated inorganic lithium bromide (LiBr) solution undergoes spontaneous aggregation into a stable gel with rapid phase-transition capability. We hypothesize that this distinct behaviour arises from an alternative denaturation mechanism that does not rely on direct interactions between proteins and concentrated ions. To investigate this, we study the denaturation effects of concentrated inorganic ion pairs using thermodynamic and spectroscopic analyses combined with atomistic molecular simulations. Through the isolation of indirect solute effects, our findings suggest a universal mechanism of salt-induced denaturation driven by entropy instead of enthalpy. We find that concentrated ion pairs like LiBr disrupt the water network structure rather than directly interacting with proteins. The mechanistic insight enables us to refine our previous extraction process of keratin materials, allowing for the spontaneous separation of denatured keratin into a condensed gel phase without additional chemicals and achieve closed-loop recycling of the LiBr denaturant. This simple, effective strategy can repurpose protein resources into versatile biomaterials in a simple, effective way without the need to separate organic denaturants from bulk proteins.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.W., J.L., E.S., and K.K.P. are inventors on a patent application filed by the President and Fellows of Harvard College (PCT application no. US2025/032176, filed June 4, 2025) titled “Sustainable protein waste regeneration via entropy-driven denaturation”. This application covers aspects of the protein denaturation and regeneration process described in this manuscript. The remaining authors declare no competing interests.
Figures



References
-
- Hu, X., Cebe, P., Weiss, A. S., Omenetto, F. & Kaplan, D. L. Protein-based composite materials. Mater. Today15, 208–215 (2012).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

