An all-in-one pipeline for the in vitro discovery and in vivo testing of Plasmodium falciparum malaria transmission blocking drugs
- PMID: 40715087
- PMCID: PMC12297408
- DOI: 10.1038/s41467-025-62014-3
An all-in-one pipeline for the in vitro discovery and in vivo testing of Plasmodium falciparum malaria transmission blocking drugs
Abstract
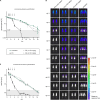
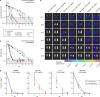
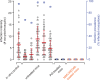
Elimination of malaria will require new drugs with potent activity against Plasmodium falciparum mature stage V gametocytes, the only stages infective to the mosquito vector. The identification and comprehensive validation of molecules active against these quiescent stages is difficult due to the specific biology of gametocytes, challenges linked to their cultivation in vitro and the lack of animal models suitable for evaluating the transmission-blocking potential of drug candidates in vivo. Here, we present a transmission-blocking drug discovery and development platform that builds on transgenic NF54/iGP1_RE9Hulg8 parasites engineered to conditionally produce large numbers of stage V gametocytes expressing a red-shifted firefly luciferase viability reporter. Besides developing a robust in vitro screening assay for the reliable identification of stage V gametocytocidal compounds, we also establish a preclinical in vivo malaria transmission model based on infecting female humanized NODscidIL2Rγnull mice with pure NF54/iGP1_RE9Hulg8 stage V gametocytes. Using whole animal bioluminescence imaging, we assess the in vivo gametocyte killing and clearance kinetics of antimalarial reference drugs and clinical drug candidates and identify markedly different pharmacodynamic response profiles. Finally, we combine this mouse model with mosquito feeding assays and thus firmly establish a valuable tool for the systematic in vivo evaluation of transmission-blocking drug efficacy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.L. is an employee of Medicines for Malaria Venture (MMV). B.B. and M.D. are employees of the Global Antibiotic Research & Development Partnership (GARDP) and were employed at MMV at the time of conceptualization and execution of the work. The remaining authors declare no competing interests.
Figures




Similar articles
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated.
-
Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Feb 02;2:CD008152. doi: 10.1002/14651858.CD008152.pub5. PMID: 25693791 Free PMC article. Updated.
-
An improved multiplex RT-quantitative PCR assay can reveal sex-specific activity of transmission-blocking drugs on ex vivo gametocytes from Plasmodium falciparum asymptomatic infections.J Antimicrob Chemother. 2025 Jul 1;80(7):1907-1914. doi: 10.1093/jac/dkaf146. J Antimicrob Chemother. 2025. PMID: 40405864 Free PMC article.
-
Primaquine or other 8-aminoquinolines for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2018 Feb 2;2(2):CD008152. doi: 10.1002/14651858.CD008152.pub5. Cochrane Database Syst Rev. 2018. PMID: 29393511 Free PMC article.
References
-
- World Health Organisation. World Malaria Report 2023. WHO Press (2023).
MeSH terms
Substances
Grants and funding
- BSCGI0_157729/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_200683/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- NCCR Chemical Biology/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- NCCR Chemical Biology/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- ANR-2019-ANR-20-CE18-0006/Agence Nationale de la Recherche (French National Research Agency)
- ANR-2019-ANR-20-CE18-0006/Agence Nationale de la Recherche (French National Research Agency)
- ERC-CoG 864180 QUANTUM/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 864.13.009/Netherlands Organisation for Scientific Research | Nationaal Regieorgaan Praktijkgericht Onderzoek SIA (Nationaal Regieorgaan Praktijkgericht Onderzoek)
- PO17/00583/Medicines for Malaria Venture (MMV)
LinkOut - more resources
Full Text Sources

