Distinct role of claustrum and anterior cingulate cortex bidirectional circuits in methamphetamine taking and seeking
- PMID: 40715089
- PMCID: PMC12297524
- DOI: 10.1038/s41467-025-62188-w
Distinct role of claustrum and anterior cingulate cortex bidirectional circuits in methamphetamine taking and seeking
Abstract
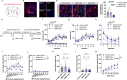
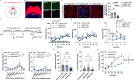
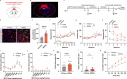
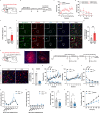
Methamphetamine (METH) addiction involves escalating intake with strong cue reactivity, and high relapse risk, yet its neural mechanism remains unclear. Using c-Fos mapping and machine learning, we identified the claustrum (CLA), a subcortical region reciprocally connected with the anterior cingulate cortex (ACC), as key mediators of both METH taking and seeking in self-administering male rats. Chemogenetic inhibition of CLA suppressed both drug consumption and cue-induced reinstatement, while ACC inhibition selectively reduced drug-seeking. Circuit tracing and manipulation revealed that the CLA-ACC circuit supported drug-taking, whereas the ACC-CLA circuit was specifically recruited during drug-seeking. Activity-dependent labeling showed that ACC ensembles activated by cues overlapped with those engaged during prior drug use. These findings suggest that CLA drives METH reward through the ACC, while the ACC gains cue salience and feeds back to CLA, reinforcing relapse. Targeting this bidirectional CLA-ACC circuit may provide novel therapeutic strategies for treating METH addiction.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- World Drug Report 2024. (United Nations publication, 2024).
-
- Huang, M. C. et al. Risk factors for relapse among methamphetamine users receiving a joint legal-medical treatment program as a diversion intervention: A one-year follow-up study. J. Subst. Use Addict. Treat.151, 208955 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

