125Te and 57Fe nuclear resonance vibrational spectroscopic characterization of intermediate spin state mixed-valent dimers
- PMID: 40715092
- PMCID: PMC12297287
- DOI: 10.1038/s41467-025-62118-w
125Te and 57Fe nuclear resonance vibrational spectroscopic characterization of intermediate spin state mixed-valent dimers
Abstract
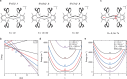
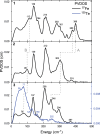
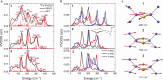
Iron-sulfur clusters fulfill numerous roles throughout biology. The reduced [2Fe-2S]+ cluster offers unique electronic and magnetic properties due to its mixed-valent nature and can serve as an essential model for understanding electron transfer, electron delocalization, and accessible spin states not only in mixed-valent dimers, but potentially larger iron sulfur clusters. Recently a series of mixed-valent diiron dichalcogenide complexes [L2Fe2Q2]- (Q = S (1), Se (2), Te (3), L = 2,6-diisopropylphenyl β-diketiminate ligand) were synthesized and characterized, where complex 1 showed a typical S = 1/2 spin state, while complexes 2 and 3 exhibited intermediate S = 3/2 spin states, potentially enabled by the minimization of vibronic coupling. Here we studied the vibrational dynamics of the Fe and Te centers in these complexes using 57Fe and 125Te nuclear resonance vibrational spectroscopy (NRVS), coupled with DFT calculations. The findings suggest that heavy character of larger chalcogen atoms results in decreased vibronic coupling. The observation of an intermediate spin state is shown to be unattainable for lighter Fe2Q2 cores. This highlights the crucial role of vibronic coupling in modulating the electronic structure of mixed-valence systems and should enhance understanding of the electronic structure in more complex biological Fe-S clusters.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Beinert, H., Holm, R. H. & Munck, E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science277, 653–659 (1997). - PubMed
-
- Solomon, E. I., Xie, X. J. & Dey, A. Mixed valent sites in biological electron transfer. Chem. Soc. Rev.37, 623–638 (2008). - PubMed
-
- Albers, A. et al. The complete characterization of a reduced biomimetic [2Fe-2S] cluster. Angew. Chem. Int. Edit. 50, 9191–9194 (2011). - PubMed
-
- Albers, A., Bayer, T., Demeshko, S., Dechert, S. & Meyer, F. A Functional model for the Rieske center: full characterization of a biomimetic N-Ligated [2Fe-2S] cluster in different protonation states. Chem. Eur. J. 19, 10101–10106 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

