Extended insight into the catalytic activity of boron-doped graphitic carbon nitride for the synthesis of bis-pyrazolyl methanes and pyranopyrazoles
- PMID: 40715186
- PMCID: PMC12297279
- DOI: 10.1038/s41598-025-11187-4
Extended insight into the catalytic activity of boron-doped graphitic carbon nitride for the synthesis of bis-pyrazolyl methanes and pyranopyrazoles
Abstract
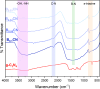
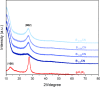
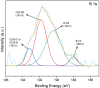
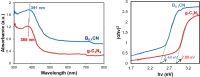
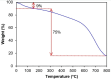
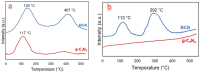
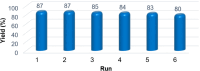
In this study, boron-doped graphitic carbon nitride (BCN) was successfully prepared via thermal copolymerization of dicyandiamide and boric acid. The structural and morphological features of the as-prepared BCN were thoroughly characterized by various physicochemical techniques such as Fourier transform infrared spectroscopy (FT-IR), powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-Vis Diffuse Reflectance Spectroscopy (UV-DRS), Brunauer-Emmett-Teller (BET) surface area analysis, and thermogravimetric analysis (TGA). The catalytic performance of BCN was then exploited in the heterogeneous multicomponent synthesis of bis(pyrazolyl)methanes and pyranopyrazoles. The superior catalytic activity of the catalyst stemmed from the B doping and N species located at the B-N-C sites, which impart acid-base dual functionality to the catalyst. These findings substantiate the pioneering utilization of BCN as a robust acid-base cooperative catalyst for the multicomponent synthesis of structurally diverse heterocycles. To the best of our knowledge, this is the first report demonstrating the use of BCN as a dual-function catalyst in MCR chemistry, opening new avenues for green and sustainable organic transformations.
Keywords: BCN; Bis-pyrazolyl methane; Boron doping; Carbon nitride; Dual function; Pyranopyrazoles.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
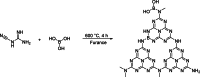


Similar articles
-
Unveiling the Favorable Synergy of ZIF-9 and Borocarbonitride on rGO as a Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reactions in Alkaline Media.Langmuir. 2025 Aug 12;41(31):20734-20745. doi: 10.1021/acs.langmuir.5c02189. Epub 2025 Jul 30. Langmuir. 2025. PMID: 40739797
-
Alginate/CuO-gC3N4 composite: a novel, reusable, non-toxic photocatalyst for methylene blue degradation.Environ Sci Pollut Res Int. 2025 Jun;32(28):16912-16930. doi: 10.1007/s11356-024-35227-0. Epub 2024 Oct 7. Environ Sci Pollut Res Int. 2025. PMID: 39373839
-
Periodic mesoporous organosilica functionalized with p-aminobenzenesulfonic acid: an efficient solid acid catalyst for one-pot synthesis of 5-substituted 1H-tetrazoles under eco-friendly conditions.Nanoscale Adv. 2025 May 19;7(15):4691-4709. doi: 10.1039/d5na00224a. eCollection 2025 Jul 22. Nanoscale Adv. 2025. PMID: 40575618 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Efficacy of nicergoline in dementia and other age associated forms of cognitive impairment.Cochrane Database Syst Rev. 2001;2001(4):CD003159. doi: 10.1002/14651858.CD003159. Cochrane Database Syst Rev. 2001. PMID: 11687175 Free PMC article.
References
-
- Allen, M. J., Tung, V. C. & Kaner, R. B. Honeycomb carbon: A review of graphene. Chem. Rev.110, 132–145 (2010). - PubMed
-
- Torrisi, F. & Coleman, J. N. Electrifying inks with 2D materials. Nat. Nanotech. 9, 738–739 (2014). - PubMed
-
- Bao, X. et al. Band structure engineering in 2D materials for optoelectronic applications. Adv. Mater. Technol.3, 1800072 (2018).
-
- Wang, J. & Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: preparation, modification and environmental application. Coord. Chem. Rev.453, 214338 (2022).
-
- Molaei, M. J., Younas, M. & Rezakazemi, M. A comprehensive review on recent advances in Two-Dimensional (2D) hexagonal Boron nitride, ACS appl. Electron. Mater.3, 5165–5187 (2021).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

