Mucosomes as next-generation drug carriers for treating mucus-resident bacterial infections and biofilms
- PMID: 40715188
- PMCID: PMC12297565
- DOI: 10.1038/s41598-025-10496-y
Mucosomes as next-generation drug carriers for treating mucus-resident bacterial infections and biofilms
Abstract
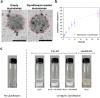
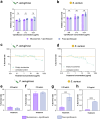
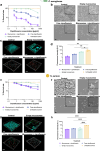
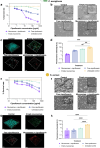
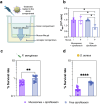
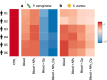
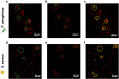
Deaths connected to bacterial infections are expected to outnumber those caused by cancer by 2050. Multiple advantages, including enhanced efficacy of the treatment, characterize the use of nanocarriers to deliver antibiotics. This work explores the use of mucosomes - intrinsically glycosylated mucin nanoparticles - to deliver ciprofloxacin to fight Pseudomonas aeruginosa and Staphylococcus aureus infections. Mucins are a family of glycoproteins representing the major non-aqueous component of human mucus and are known for actively interacting with bacteria, reducing their virulence, and limiting their aggregations. This study shows that these critical properties of mucin are preserved in mucosomes, enabling a strong synergy with the loaded antimicrobial drug. Empty mucosomes exert a bacteriostatic activity, inhibiting bacterial growth up to 70%. Ciprofloxacin-loaded mucosomes were able to decrease the minimum inhibitory concentration of ciprofloxacin against S. aureus by up to 50%. Mucosomes could prevent biofilm formation and disassemble well-established biofilms by reducing the biomass by up to 98%. Mucosomes further facilitated the transmucosal delivery of ciprofloxacin in a 3D mucus-mimicking model. These results, together with the possibility of freeze-drying and storing drug-loaded mucosomes without impairing their efficacy, suggest the suitability of this approach to tackle mucosal bacterial infections. Interestingly, this nanosystem has been shown to enhance the phagocytic action of blood in eradicating bacterial biofilms.
Keywords: Ciprofloxacin; Drug delivery; Glycans; Infections; Mucin; Mucosa; Nanoparticles.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: P.P., L.V., and S.V. are co-founders and Scientific advisors of Bac3Gel lda., Portugal, and are co-inventors of the patent IT102018000020242A “Three-dimensional substrate for microbial cultures”. P.P., L.V., and S.V. are co-inventors of the patent IT102020000014908A “Covalently cross-linked glycosylated mucin nanoparticles as systems for the delivery and release of active ingredients and biomolecules”. The authors declare no other competing interests. Ethics: The experiments described in Sect. “Hemolytic activity” and "Phagocytic assay of mucosome-treated bacterial biofilms" included the use of biological materials from human healthy donors. Human blood obtained from healthy donors was provided by Fondazione IRCCS Policlinico San Matteo, Pavia (Italy), and isolated according to Italian national policies, including “Decreto Ministero della Salute 2 November 2015 n.69” and “Accordo Stato-Regioni n.225/CSR 13 December 2018”. The study and all experimental protocols were approved by the Ethics Committee of Fondazione IRCCS Policlinico San Matteo, Pavia (approval number not applicable). All methods were performed in accordance with the relevant guidelines and regulations. Per the Declaration of Helsinki, informed consent was obtained from all donors prior to sample collection.
Figures

Similar articles
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2014 Nov 10;(11):CD004197. doi: 10.1002/14651858.CD004197.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Apr 25;4:CD004197. doi: 10.1002/14651858.CD004197.pub5. PMID: 25383937 Updated.
-
Wound healing properties and antibiofilm activity of hydrogel matrix containing nitric oxide, silver nanoparticles, and ciprofloxacin against Pseudomonas aeruginosa burn wound infection.Sci Rep. 2025 Jul 21;15(1):26508. doi: 10.1038/s41598-025-12385-w. Sci Rep. 2025. PMID: 40691256 Free PMC article.
-
Copper-coated carbon-infiltrated carbon nanotube surfaces effectively inhibit Staphylococcus aureus and Pseudomonas aeruginosa biofilm formation.Appl Environ Microbiol. 2025 Jul 8:e0105325. doi: 10.1128/aem.01053-25. Online ahead of print. Appl Environ Microbiol. 2025. PMID: 40626868
-
Enhanced therapeutic efficacy of silibinin loaded silica coated magnetic nanocomposites against Pseudomonas aeruginosa in Combination with Ciprofloxacin and HepG2 cancer cells.Sci Rep. 2025 Jul 1;15(1):21498. doi: 10.1038/s41598-025-07529-x. Sci Rep. 2025. PMID: 40596538 Free PMC article.
References
-
- Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nature Reviews Microbiology 2022 21:5 21, 280–295 (2022). - PubMed
-
- MacNair, C. R., Rutherford, S. T. & Tan, M. W. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nature Reviews Microbiology 2023 22:5 22, 262–275 (2023). - PubMed
-
- Brown, D. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nature Reviews Drug Discovery 2015 14:12 14, 821–832 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

