Dual α-amylase and α-glucosidase inhibition by 1,2,4-triazole derivatives for diabetes treatment
- PMID: 40715234
- PMCID: PMC12297423
- DOI: 10.1038/s41598-025-11214-4
Dual α-amylase and α-glucosidase inhibition by 1,2,4-triazole derivatives for diabetes treatment
Erratum in
-
Correction: Dual α-amylase and α-glucosidase inhibition by 1,2,4-triazole derivatives for diabetes treatment.Sci Rep. 2025 Aug 15;15(1):29950. doi: 10.1038/s41598-025-15989-4. Sci Rep. 2025. PMID: 40817359 Free PMC article. No abstract available.
Abstract
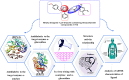
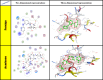
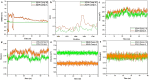
The development of effective antidiabetic agents remains a critical challenge in diabetes management. In this study, we introduce novel 1,2,4-triazole-based derivatives designed as dual inhibitors of α-amylase and α-glucosidase, key enzymes in carbohydrate metabolism. Molecular docking identified six promising candidates, with compounds 4 and 10 showing the highest potency. Both compounds exhibited strong α-glucosidase inhibition (IC50 = 0.27 ± 0.01 µg/mL and 0.31 ± 0.01 μg/mL, respectively), surpassing acarbose, and also demonstrated potent α-amylase inhibition (IC50 = 0.19 ± 0.01 μg/mL and 0.26 ± 0.01 μg/mL, respectively). Structure-activity relationship analysis highlighted the crucial role of acetyl and bromo substituents in enhancing enzyme inhibition. These findings position triazole-based scaffolds as promising candidates for the development of next-generation antidiabetic therapies.
Keywords: 1,2,4-Triazole derivatives; Anti-diabetic activity; In vitro enzyme inhibition; Molecular docking analysis; α-Amylase inhibitors; α-Glucosidase inhibitors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors certify that none of the research provided in this paper has been impacted by any known financial or interpersonal conflicts.
Figures










References
-
- Marzouk, M. A. Pyrimidine derivatives as multifaceted antidiabetic agents: A comprehensive review of structure-activity relationships, mechanisms, and clinical potential. Eur. J. Med. Chem.296, 117859. 10.1016/j.ejmech.2025.117859 (2025). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

