In vitro and in silico neuroprotective evaluation of new biotransformation metabolites of (-)-α-bisabolol
- PMID: 40715238
- PMCID: PMC12297533
- DOI: 10.1038/s41598-025-11694-4
In vitro and in silico neuroprotective evaluation of new biotransformation metabolites of (-)-α-bisabolol
Abstract
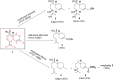
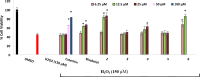
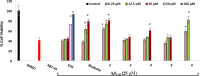
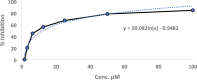
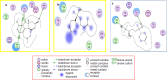
Biotransformation of (-)-α-bisabolol (1) was investigated by screening twenty-two fungal strains in an effort to produce new more polar and potentially bioactive metabolites. Three fungi were selected for scale-up biotransformation: Cordyceps sinensis, Alternaria alternata and Aspergillus flavus. Five metabolites were isolated: 10β,11-dihydroxy-α-bisabolol (2), Hamanasic acid A (3), 2,3-dihydro-α-bisabolol (4), 7-dehydroxy-10,11-epoxy-3-methylcarboxy-α-bisabolol (5) and 10β,11,15-trihydroxy-α-bisabolol (6), with metabolites 4, 5, and 6 being newly identified. Structural elucidation was performed using spectroscopic methods. α-bisabolol and its metabolites were evaluated for their cyclooxygenase (COX) and acetylcholinesterase (AChE) inhibitory activities, as well as neuroprotective effects against H2O2 and Aβ1-42-induced toxicity in SH-SY5Y cells. In vitro results showed that metabolite 5 exhibited the strongest COX-2 inhibition (IC50 = 2.508 µM), while 2 showed AChE inhibition (IC50 = 12.94 µM), These outcomes were more confirmed by molecular docking. Metabolites 6 and 2 demonstrated superior neuroprotective effects against H2O2 and Aβ1-42-induced toxicity compared to α-bisabolol. Importantly, metabolite 2 showed pronounced AChE inhibitory activity alongside favorable ADMET attributes. These findings suggest that α-bisabolol and its metabolite 2 are potential candidates for the modulation of neurodegenerative diseases involving inflammation, neurotoxicity, or cholinergic dysfunction. Further in vivo investigations are mandatory to ensure the study outcomes.
Keywords: Biotransformation; Bisabolol; COX inhibitors; Cholinesterase inhibitors; Hamanasic acid A; Neuroprotective.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
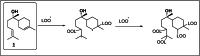
Similar articles
-
In Vitro Assessment of Cholinesterase Inhibition and Neuroprotective Effects of Elaeocarpus angustifolius Blume Against Amyloid-Beta Peptide-Induced Toxicity in SH-SY5Y and BV-2 Cells.Neurochem Res. 2025 Jul 9;50(4):226. doi: 10.1007/s11064-025-04478-9. Neurochem Res. 2025. PMID: 40632335
-
Design, synthesis, and biological evaluation of caffeic acid-based novel multifunctional molecules for the management of Alzheimer's disease.Eur J Med Chem. 2025 Oct 15;296:117831. doi: 10.1016/j.ejmech.2025.117831. Epub 2025 Jun 10. Eur J Med Chem. 2025. PMID: 40578253
-
Synthesis, Biological Evaluation, Molecular Docking, and In Silico ADME Predictions of Huperzine: A Derivative for the Novel Protective Application Against Neurodegenerations.Chem Asian J. 2025 Jun;20(12):e202401950. doi: 10.1002/asia.202401950. Epub 2025 Apr 7. Chem Asian J. 2025. PMID: 40195677
-
Interventions for preventing neuropathy caused by cisplatin and related compounds.Cochrane Database Syst Rev. 2014 Mar 31;2014(3):CD005228. doi: 10.1002/14651858.CD005228.pub4. Cochrane Database Syst Rev. 2014. PMID: 24687190 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Kamatou, G. P. & Viljoen, A. M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc.87(1), 1–7 (2010).
-
- Russell, K. & Jacob, S. E. Bisabolol. Dermatitis®21(1), 57–58 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

