Circulating biomarkers in patients with progressive fibrosing interstitial lung disease treated with nintedanib: a pilot study
- PMID: 40715322
- PMCID: PMC12297266
- DOI: 10.1038/s41598-025-12952-1
Circulating biomarkers in patients with progressive fibrosing interstitial lung disease treated with nintedanib: a pilot study
Abstract
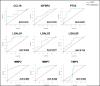
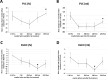
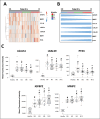
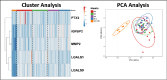
Progressive fibrosing interstitial lung diseases (PF-ILDs) are characterized by persistent progression and have limited treatment options. The identification of reliable biomarkers to monitor fibrosis and therapeutic response remains a clinical challenge. This study investigated circulating plasma biomarkers associated with PF-ILDs and their potential role in monitoring disease evolution during nintedanib treatment. From 127 putative fibrosis biomarkers, seven candidates were identified with high diagnostic value (area under the curve [AUC] > 0.7), of which five (IGFBP2, PTX3, LGALS1, LGALS9, and MMP2) showed significant dynamic changes (assessed by longitudinal plasma proteomic analysis) in PF-ILD patients treated with 12-months nintedanib, correlating with improvements in forced vital capacity and diffusing capacity of the lung for carbon monoxide. Principal component analysis identified a shift in molecular profiles over time, suggesting nintedanib-induced modulation of these biomarkers. Receiver operating characteristic analysis demonstrated that while LGALS9 maintained a stable predictive value during nintedanib treatment, LGALS1, IGFBP2, PTX3, and MMP2 exhibited increasing AUC scores, indicating their potential role in monitoring fibrosis progression. We also identified optimal biomarker cut-off values at 12 months, which may provide reliable thresholds for fibrosis assessment. In conclusion, our exploratory analysis identified five biomarkers whose plasma concentrations changed during antifibrotic treatment, highlighting their potential prognostic value. Further validation in larger cohorts is needed to confirm their clinical utility.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The study was conducted in accordance with the Declaration of Helsinki. Samples were obtained with fully informed written consent, and ethics approval was obtained from the IRCCS ISMETT’s Institutional Research Review Board supervisors (project number: IRRB/08/22) and the relevant local ethics committee.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

