Monoclonal antibody for phosphorylated TRIO Y2681 that helps predict prognosis of post-operative colorectal cancer patients
- PMID: 40715467
- PMCID: PMC12297228
- DOI: 10.1038/s44276-025-00163-0
Monoclonal antibody for phosphorylated TRIO Y2681 that helps predict prognosis of post-operative colorectal cancer patients
Abstract
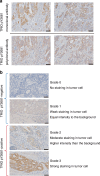
Background: We previously reported that TRIO pY2681, a novel prognostic biomarker for CRC, can be detected by polyclonal antibodies (pAb). We have now developed a novel monoclonal antibody (mAb) that recognizes TRIO pY2681. This study aims to assess the utility of immunohistochemical (IHC) staining using the TRIO pY2681 mAb as a prognostic marker for CRC in clinical practice.
Methods: IHC using TRIO pY2681 mAb was performed on surgical specimens from 357 CRC patients at Kyoto Medical Center and 320 at Kyoto University Hospital. Based on the results, we conducted a retrospective outcome analysis.
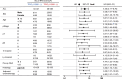
Results: TRIO pY2681 mAb exhibited significantly higher titers than pAb. In both cohorts of all stages, TRIO pY2681 IHC positivity correlated with shorter disease-specific survival (DSS) (HR, 1.67; 95% CI, 1.00-2.79; P = 0.046, and HR, 5.84; 95% CI, 2.26-15.1; P < 0.001) and relapse-free survival (RFS) (HR, 1.92; 95% CI, 1.15-3.22; P = 0.011, and HR, 4.36; 95% CI, 2.17-8.76; P < 0.001). The trend persisted in stage III. Multivariate analysis confirmed TRIO pY2681 IHC positivity as an independent prognostic factor for RFS.
Conclusions: The novel TRIO pY2681 mAb identifies CRC patient subsets with poorer prognoses, enhancing prognostic precision in clinical settings.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: MMT has ownership interest in and consultant/advisory board relationship with KyoDiagnostics KK (Kyoto, Japan) that executes patent rights owned by Kyoto University for diagnostic use of TRIO pY2681 antibodies. Other authors have no competing interests. Ethics approval and consent to participate: This study was performed in accordance with the Declaration of Helsinki. The study protocol involving human samples was approved by the Kyoto University Graduate School and Faculty of Medicine, Ethics Committee as well as the Ethics Committee of Kyoto Medical Center. All participants provided their written informed consent for study participation, including the use of their tissue samples and data analysis (Approval No. G0550, FY 2013-2024).
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669–85. - PubMed
-
- Debant A, Serra-Pagès C, Seipel K, O’Brien S, Tang M, Park SH, et al. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci USA. 1996;93:5466–71. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
